��Ŀ����
�ס��ҡ����������������ǰ20��Ԫ����ɵij������ʣ������й���Ϣ�ش��������⣺
(1)��ɫ������ɷǼ��Է��ӹ��ɣ��Ҽ�����ֻ��1�Թ��õ��Ӷԣ��ķ���ʽΪ____________��
(2)�����Ǻ��зǼ��Ե��������ӻ�������ҵ���ɫ��Ӧ�ʻ�ɫ�����ҵĵ���ʽΪ____________�������Ǻ��зǼ������������ӻ�������ҵĵ���ʽΪ______________��
(3)�����������ֳ������廯�����ɣ������ȷֽ�ʱ�ƻ��˹��ۼ������Ӽ���ȴֻ�γɹ��ۼ���д��������ˮʱ������Ӧ�����ӷ���ʽ_________________________��
(4)���ʶ�����Ҫ�İ뵼����ϣ�����������ľ���������___________��д����ҵ�Ʊ����Ļ�ѧ��Ӧ����ʽ_____________________��
(5)�죨W3Q4������Ԫ��W��Q�γɵĽṹ�մɲ��ϣ����һ�ֺϳɷ������£�W���Ȼ�����Q���⻯����ȷ�Ӧ�����ɻ�����W(QH2)4��HCl���壻W(QH2)4�ڸ����·ֽ�����Q���⻯����졣������ط�Ӧ�Ļ�ѧ����ʽ���������û�ѧʽ��ʾ����__________________��
(1)��ɫ������ɷǼ��Է��ӹ��ɣ��Ҽ�����ֻ��1�Թ��õ��Ӷԣ��ķ���ʽΪ____________��
(2)�����Ǻ��зǼ��Ե��������ӻ�������ҵ���ɫ��Ӧ�ʻ�ɫ�����ҵĵ���ʽΪ____________�������Ǻ��зǼ������������ӻ�������ҵĵ���ʽΪ______________��
(3)�����������ֳ������廯�����ɣ������ȷֽ�ʱ�ƻ��˹��ۼ������Ӽ���ȴֻ�γɹ��ۼ���д��������ˮʱ������Ӧ�����ӷ���ʽ_________________________��
(4)���ʶ�����Ҫ�İ뵼����ϣ�����������ľ���������___________��д����ҵ�Ʊ����Ļ�ѧ��Ӧ����ʽ_____________________��
(5)�죨W3Q4������Ԫ��W��Q�γɵĽṹ�մɲ��ϣ����һ�ֺϳɷ������£�W���Ȼ�����Q���⻯����ȷ�Ӧ�����ɻ�����W(QH2)4��HCl���壻W(QH2)4�ڸ����·ֽ�����Q���⻯����졣������ط�Ӧ�Ļ�ѧ����ʽ���������û�ѧʽ��ʾ����__________________��
(1)H2
(2) ��
��
(3)NH4++H2O NH3��H2O+H+
NH3��H2O+H+
(4)ԭ�Ӿ��壻2C+SiO2 Si+2CO��
Si+2CO��
(5)SiCl4+4NH3 Si(NH2)4+4HCl��3Si(NH2)4
Si(NH2)4+4HCl��3Si(NH2)4 8NH3��+Si3N4
8NH3��+Si3N4
(2)
 ��
��
(3)NH4++H2O
 NH3��H2O+H+
NH3��H2O+H+ (4)ԭ�Ӿ��壻2C+SiO2
 Si+2CO��
Si+2CO��(5)SiCl4+4NH3
 Si(NH2)4+4HCl��3Si(NH2)4
Si(NH2)4+4HCl��3Si(NH2)4 8NH3��+Si3N4
8NH3��+Si3N4 
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
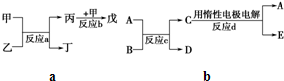
 �ס��ҡ������������Ϊ��ѧ��ѧ�����ĵ��ʻ���Ҿ�����ͬһ��Ԫ��R����һ����������ת����ϵ��ͼ��ʾ��
�ס��ҡ������������Ϊ��ѧ��ѧ�����ĵ��ʻ���Ҿ�����ͬһ��Ԫ��R����һ����������ת����ϵ��ͼ��ʾ��


 6����Ϊ��������Ҫ�ɷ�֮һ���ĵ���ʽΪ ���ҵĿռ乹��Ϊ �����ϡ��Һ�ܸ��������۷�Ӧ�������ӷ�Ӧ����ʽΪ ��
6����Ϊ��������Ҫ�ɷ�֮һ���ĵ���ʽΪ ���ҵĿռ乹��Ϊ �����ϡ��Һ�ܸ��������۷�Ӧ�������ӷ�Ӧ����ʽΪ ��