��Ŀ����
��֪��A��B��F�Ǽ�ͥ�г������л��F������ʳƷ��װ��E��ʯ�ͻ�����չˮƽ�ı�־����������ת����ϵ�ش����⡣

��1���ֱ�д��A��E�й����ŵ����ƣ�A�� ��E�� ��
(2) ����������Ϊ________________��
(3)ȡ����Ӧ�ķ���ܹ㣬�١���������ȡ����Ӧ����________(�����)��
(4)��д�����з�Ӧ�Ļ�ѧ����ʽ��
��.C��ϡ������Һ���ȷ�Ӧ______________________________________��
��.B�ڽ���ͭ�������ڿ����м��ȷ�Ӧ__________________________��
��.D��������ϡ���ᷴӦ____________________________________��
��.F�ڿ�������ȫȼ�� ��
(5)F��һ�ֳ����ĸ߷��Ӳ��ϣ��������Ǵ����˾�ķ��㡣Ȼ�������ֲ�����ɵĵ����ijһ����������________________________________________��

��1���ֱ�д��A��E�й����ŵ����ƣ�A�� ��E�� ��
(2) ����������Ϊ________________��
(3)ȡ����Ӧ�ķ���ܹ㣬�١���������ȡ����Ӧ����________(�����)��
(4)��д�����з�Ӧ�Ļ�ѧ����ʽ��
��.C��ϡ������Һ���ȷ�Ӧ______________________________________��
��.B�ڽ���ͭ�������ڿ����м��ȷ�Ӧ__________________________��
��.D��������ϡ���ᷴӦ____________________________________��
��.F�ڿ�������ȫȼ�� ��
(5)F��һ�ֳ����ĸ߷��Ӳ��ϣ��������Ǵ����˾�ķ��㡣Ȼ�������ֲ�����ɵĵ����ijһ����������________________________________________��
��1���Ȼ� ̼̼˫�� ��(2)���� (3)�٢ڢ�
(4) ��.CH3COOCH2CH3��H2O CH3CH2OH��CH3COOH�����棩
CH3CH2OH��CH3COOH�����棩
��. 2CH3CH2OH + O2 2CH3CHO��2H2O (�����������)
2CH3CHO��2H2O (�����������)
��. CH3COONa + HCl CH3COOH + NaCl
CH3COOH + NaCl
�� + 3nO2
+ 3nO2 2nCO2 + 2nH2O
2nCO2 + 2nH2O
(4)��ɫ��Ⱦ
(4) ��.CH3COOCH2CH3��H2O
 CH3CH2OH��CH3COOH�����棩
CH3CH2OH��CH3COOH�����棩��. 2CH3CH2OH + O2
 2CH3CHO��2H2O (�����������)
2CH3CHO��2H2O (�����������)��. CH3COONa + HCl
 CH3COOH + NaCl
CH3COOH + NaCl��
 + 3nO2
+ 3nO2 2nCO2 + 2nH2O
2nCO2 + 2nH2O(4)��ɫ��Ⱦ
E��ʯ�ͻ�����չˮƽ�ı�־����E����ϩ��F������ʳƷ��װ������F����ϩ�ļӾ۲������ϩ����ϩ����̼̼˫������ˮ�����ӳɷ�Ӧ�������Ҵ�������ΪAB���Ǽ�ͥ�г������л������Aˮ���ᣬ���Ҵ�����������Ӧ����C��Cˮ���ֲ���������Ҵ���
��1����
��2�����͡����͵������ܽ⣬���е���Ƚϴ�����ͨ�����ɡ�
��3���л����е�ijЩԭ�ӻ�ԭ���ű�����ԭ�ӻ�ԭ������ȡ���ķ�Ӧ��ȡ����Ӧ,���Դ��Ǣ٢ڢۡ�
��4������5���ԡ�
��1����
��2�����͡����͵������ܽ⣬���е���Ƚϴ�����ͨ�����ɡ�
��3���л����е�ijЩԭ�ӻ�ԭ���ű�����ԭ�ӻ�ԭ������ȡ���ķ�Ӧ��ȡ����Ӧ,���Դ��Ǣ٢ڢۡ�
��4������5���ԡ�

��ϰ��ϵ�д�
 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ


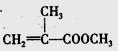 �����л������ĵ��壬������ں˴Ź�������ͼ������ʾ_________����ͬ�ķ塣�����������ڼ���ϩ�����ͬ���칹����� ____ ����ѡ����ţ���CH3COOCH2CH=CH2����CH2=C��CH2CH3��COOCH3����
�����л������ĵ��壬������ں˴Ź�������ͼ������ʾ_________����ͬ�ķ塣�����������ڼ���ϩ�����ͬ���칹����� ____ ����ѡ����ţ���CH3COOCH2CH=CH2����CH2=C��CH2CH3��COOCH3����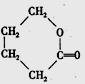 ����
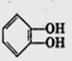
����
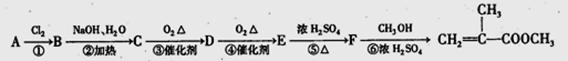

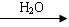 R��COOH
R��COOH

 ��
�� ��
�� �⣬
�⣬ ���ȶ�)��
���ȶ�)��


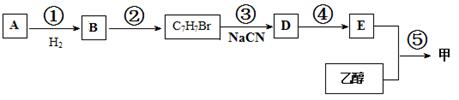
 ���ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ��Ϊ��
���ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ��Ϊ��
