��Ŀ����
ij̽��С��ͨ���Ƚ�����KMnO4��Һ��H2C2O4��Һ��Ӧ�����У���Һ��ɫ��ʧʱ�䳤�̵ķ������о�Ũ�ȡ��¶ȶԷ�Ӧ���ʵ�Ӱ�죮
�ɹ�ѡ���ʵ��������KMnO4������Һ��Ũ�ȿ�ѡ��0.01mol?L-1��0.1mol?L-1��H2C2O4��Һ��Ũ�ȿ�ѡ��0.1mol?L-1��0.2mol?L-1��ʵ���¶ȿ�ѡ��298K��323K��ÿ��ʵ��KMnO4������Һ��������Ϊ4mL��H2C2O4��Һ��������Ϊ2mL��
��1��ʵ����ƣ����������ʵ����Ʊ���
��2��ʵ���¼��ʵ������Ҫ��¼��������
�ɹ�ѡ���ʵ��������KMnO4������Һ��Ũ�ȿ�ѡ��0.01mol?L-1��0.1mol?L-1��H2C2O4��Һ��Ũ�ȿ�ѡ��0.1mol?L-1��0.2mol?L-1��ʵ���¶ȿ�ѡ��298K��323K��ÿ��ʵ��KMnO4������Һ��������Ϊ4mL��H2C2O4��Һ��������Ϊ2mL��
��1��ʵ����ƣ����������ʵ����Ʊ���
| ʵ���� | T/K | KMnO4Ũ�� /mol?L-1 |
H2C2O4Ũ�� /mol?L-1 |
ʵ��Ŀ�� |
| �� | 298 | 0.01mol?L-1 | 0.1mol?L-1 | Ϊ����ʵ�������� |
| �� | 323 323 |
0.01 mol?L-1 0.01 mol?L-1 |
0.1 mol?L-1 0.1 mol?L-1 |
̽���¶ȶԷ�Ӧ���ʵ�Ӱ�� ̽���¶ȶԷ�Ӧ���ʵ�Ӱ�� |
| �� | 298 298 |
0.01 mol?L-1 0.01 mol?L-1 |
0.2 mol?L-1 0.2 mol?L-1 |
̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
����ʵ������Һ��ɫ��ʱ��
����ʵ������Һ��ɫ��ʱ��
����������1����������֪��ʵ��Ŀ����̽��Ũ�ȡ��¶ȶԷ�Ӧ���ʵ�Ӱ�죬���Եڶ���ʵ�����һ��ʵ����������¶ȣ�����������ͬ��������ʵ����̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬���Ե��������¶Ⱥ͵�һ����ͬ����Ӧ���Ũ�Ȳ�ͬ��
��2������v=
ȷ����Ҫ��¼�����ݣ�
��2������v=
| ��c |
| ��t |
����⣺��1����������֪��ʵ��Ŀ����̽��Ũ�ȡ��¶ȶԷ�Ӧ���ʵ�Ӱ�죬��֪������ʵ��Ŀ����̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬���Եڶ���ʵ����̽���¶ȶԷ�Ӧ���ʵ�Ӱ�죬��ڶ���ʵ��͵�һ��ʵ��IJ�ͬ����ʵ���¶��ϣ�����������ͬ�����Ե�����ʵ����̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬���������¶Ⱥ͵�һ����ͬ����Ӧ���Ũ�Ȳ�ͬ��
�ʴ�Ϊ��
��˵������Ϊ��ͨ���Ƚ�KMnO4��ɫʱ�䳤�����жϷ�Ӧ���ʵĿ�����ʵ����н��ɸı�H2C2O4��Ũ�ȣ������ܸı�KMnO4��Ũ�ȣ����⣬ѡ��Ũ��Ϊ0.1 mol?L-1 KMnO4����������۲첻����Һ��ɫ������
��2������v=
֪��ʵ������Ҫ��¼�������Ǹ���ʵ������Һ��ɫ��ʱ�䣻
�ʴ�Ϊ������ʵ������Һ��ɫ��ʱ�䣮
�ʴ�Ϊ��
| ʵ���� | T/K | KMnO4Ũ�� /mol?L-1 |
H2C2O4Ũ�� /mol?L-1 |
ʵ��Ŀ�� |
| �� | ||||
| �� | 323 | 0.01 mol?L-1 | 0.1 mol?L-1 | ̽���¶ȶԷ�Ӧ���ʵ�Ӱ�� |
| �� | 298 | 0.01 mol?L-1 | 0.2 mol?L-1 |
��2������v=
| ��c |
| ��t |
�ʴ�Ϊ������ʵ������Һ��ɫ��ʱ�䣮
���������⿼����̽���¶ȡ�Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬ע��ʵ����н��ɸı�H2C2O4��Ũ�ȣ������ܸı�KMnO4��Ũ�ȣ������Ѷ��еȣ�

��ϰ��ϵ�д�
 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
�����Ŀ
��I���ζ��������ǻ�ѧ�������е���Ҫ��������֮һ��
��һ��������к͵ζ����ⶨ���۰״���������g/100mL����
A��ʵ�鲽�裺
��1������ʽ�ζ�����ȡ10.00mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL����ƿ�ж��ݣ�ҡ�ȼ��ô���״���Һ��
��2������ʽ�ζ���ȡ����״���Һ20.00mL����ƿ�У������еμ�2�η�̪��ָʾ����
��3����ȡʢװ0.1000mol/L NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ______��
��4���ζ�����______ʱ��
ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Σ�
B��ʵ���¼
C�����ݴ��������ۣ�
��1�������㣬���۰״�������=______g/100mL��
��2���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����______����д��ţ���
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
��������˫ָʾ�����ⶨ����ĺ���
��ͼ�ĵζ����߱�ʾ����0.1000mol/L HCl�ζ� 20.00ml 0.1000mol/L Na2CO3

��A��ѡ��______��ָʾ���ȽϺ��ʣ�
�ڵ��ζ������ҺpH�ӽ�4ʱ��Ϊ�˷�ֹ�յ���ֹ��磬����ʵ����Ӧ����ҡ����Һ�����һ����Һ����ȴ���ټ����ζ����������������ǣ�______��
��II������MnO2����H2O2��Һ�ֽ�Ĵ�����ijУ��ѧ��ȤС����̽������һЩ�����������Ƿ�Ҳ������H2O2��Һ�ֽ�Ĵ�����̽���������£�
��1��[����]��Al2O3������������ֽ�Ĵ�����
��2��[ʵ����֤]��
��3��[����]����Al2O3����H2O2��Һ�ֽ�Ĵ�����
��4��[�����뷴˼]���е�ͬѧ��Ϊֻ����������ʵ�飬������ȫ֤��Al2O3��H2O2��Һ�ֽ������˴����ã���Ӧ����ʵ������֤��______��
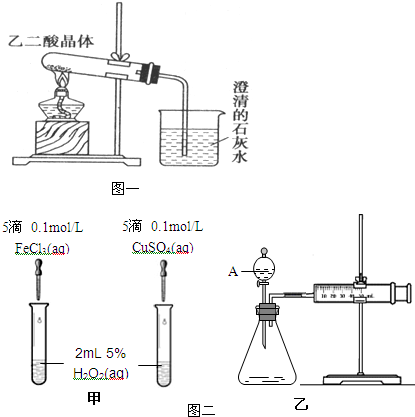
��5�����⻯ѧ��ȤС��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч��������ͬѧ�������ͼ��ʾ��ʵ�飮��ͨ���۲췴Ӧ�������ݿ�����Ӧ��ɵ��Ⱥ���Թܱڵ����ȳ̶ȶ��ԱȽϵó����ۣ���ͬѧ�����CuSO4��ΪCuCl2��Ϊ��������������______������Ϊ���������θĽ���______��


��һ��������к͵ζ����ⶨ���۰״���������g/100mL����
A��ʵ�鲽�裺
��1������ʽ�ζ�����ȡ10.00mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL����ƿ�ж��ݣ�ҡ�ȼ��ô���״���Һ��
��2������ʽ�ζ���ȡ����״���Һ20.00mL����ƿ�У������еμ�2�η�̪��ָʾ����
��3����ȡʢװ0.1000mol/L NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ______��
��4���ζ�����______ʱ��
ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Σ�
B��ʵ���¼
| �ζ����� ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.95 | 15.00 | 15.05 | 14.95 |
��1�������㣬���۰״�������=______g/100mL��
��2���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����______����д��ţ���
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
��������˫ָʾ�����ⶨ����ĺ���
��ͼ�ĵζ����߱�ʾ����0.1000mol/L HCl�ζ� 20.00ml 0.1000mol/L Na2CO3

��A��ѡ��______��ָʾ���ȽϺ��ʣ�
�ڵ��ζ������ҺpH�ӽ�4ʱ��Ϊ�˷�ֹ�յ���ֹ��磬����ʵ����Ӧ����ҡ����Һ�����һ����Һ����ȴ���ټ����ζ����������������ǣ�______��
��II������MnO2����H2O2��Һ�ֽ�Ĵ�����ijУ��ѧ��ȤС����̽������һЩ�����������Ƿ�Ҳ������H2O2��Һ�ֽ�Ĵ�����̽���������£�
��1��[����]��Al2O3������������ֽ�Ĵ�����
��2��[ʵ����֤]��
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| ʵ��һ | �������ǵ�ľ������װ�й���������Һ���Թ��� | ľ������ȼ | �����¹���������Һ���ֽ⣨��ֽ���٣�______ |
| ʵ��� | ��װ��H2O2��Һ���Թ��м�������Al2O3��Ȼ�����ǵ�ľ�������Թ��� | ľ����ȼ | ______ |
��4��[�����뷴˼]���е�ͬѧ��Ϊֻ����������ʵ�飬������ȫ֤��Al2O3��H2O2��Һ�ֽ������˴����ã���Ӧ����ʵ������֤��______��
��5�����⻯ѧ��ȤС��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч��������ͬѧ�������ͼ��ʾ��ʵ�飮��ͨ���۲췴Ӧ�������ݿ�����Ӧ��ɵ��Ⱥ���Թܱڵ����ȳ̶ȶ��ԱȽϵó����ۣ���ͬѧ�����CuSO4��ΪCuCl2��Ϊ��������������______������Ϊ���������θĽ���______��


 ��I���ζ��������ǻ�ѧ�������е���Ҫ��������֮һ��
��I���ζ��������ǻ�ѧ�������е���Ҫ��������֮һ��