��Ŀ����
����ѹǿ����������ʵ��ͻ�ѧʵ��������ҪӰ�죬��ȡ�����������Ȫʵ�飮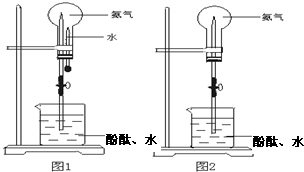
��1��ʵ���ҿ����Ȼ������ʯ����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��
��2���ռ�����Ӧʹ��
��3����ͼ1װ�ý�����Ȫʵ�飬�ϲ���ƿ��װ�����ﰱ��������ˮ����IJ�����
��4�����ֻ�ṩ��ͼ2��װ�ã���˵��������Ȫ�ķ���
��������1��ʵ��������ʯ�Һ��Ȼ���ڼ��������·�Ӧ�Ʊ�������
��2�����ݰ�������������ѡ���ռ����������ݰ���ˮ��Һ�ʼ���ѡ����﷽���ͼ��鷽����
��3�����ð�����������ˮ���γ�ѹǿ����γ���Ȫ��
��4������ʱ��ƿ��ѹǿ��������������ͣ���������ˮ�Ӵ�ʱ�ᵼ����ƿ��ѹǿ��С��
��2�����ݰ�������������ѡ���ռ����������ݰ���ˮ��Һ�ʼ���ѡ����﷽���ͼ��鷽����
��3�����ð�����������ˮ���γ�ѹǿ����γ���Ȫ��
��4������ʱ��ƿ��ѹǿ��������������ͣ���������ˮ�Ӵ�ʱ�ᵼ����ƿ��ѹǿ��С��
����⣺��1��ʵ��������ʯ�Һ��Ȼ���ڼ��������·�Ӧ�Ʊ���������Ӧ�Ļ�ѧ����ʽΪCa��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O��
��2��������������ˮ����������ˮ���ռ����ܶȱȿ���С���������ſ������ռ�������Ϊ�������壬�ü�ʯ�Ҹ������ʪ��ĺ�ɫʯ����ֽ���鰱���������ǽ�ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ��������˵�������ռ�����
�ʴ�Ϊ�������ſ�������ʯ�ң���ʪ��ĺ�ɫʯ����ֽ�����Թܿڱ�����
��3��������������ˮ�������ֹˮ�У���������ˮ����ƿ��ѹǿѸ�ټ�С�����γ���Ȫ��������ˮ��Ӧ���ɰ�ˮ����ˮ�ܵ����笠����Ӻ����������ӣ�������Һ�ʼ��ԣ���̪��Һ������ɫ���漰��ӦΪNH3+H2O?NH3?H2O?NH4++OH-��
�ʴ�Ϊ����ֹˮ�У�������ͷ�ι��е�ˮ��NH3+H2O?NH3?H2O?NH4++OH-��
��4������ʱ��ƿ��ѹǿ��������������ͣ���������ˮ�Ӵ�ʱ��������������ˮ��������ƿ��ѹǿѸ�ټ�С���γ���Ȫ��
�ʴ�Ϊ�����ӣ����֣�����ë���ȣ�����ƿ���ȣ����������������ͣ��ϳ����������ڵĿ�����������ˮ�Ӵ�����������Ȫ��
| ||
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
| ||
��2��������������ˮ����������ˮ���ռ����ܶȱȿ���С���������ſ������ռ�������Ϊ�������壬�ü�ʯ�Ҹ������ʪ��ĺ�ɫʯ����ֽ���鰱���������ǽ�ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ��������˵�������ռ�����
�ʴ�Ϊ�������ſ�������ʯ�ң���ʪ��ĺ�ɫʯ����ֽ�����Թܿڱ�����
��3��������������ˮ�������ֹˮ�У���������ˮ����ƿ��ѹǿѸ�ټ�С�����γ���Ȫ��������ˮ��Ӧ���ɰ�ˮ����ˮ�ܵ����笠����Ӻ����������ӣ�������Һ�ʼ��ԣ���̪��Һ������ɫ���漰��ӦΪNH3+H2O?NH3?H2O?NH4++OH-��
�ʴ�Ϊ����ֹˮ�У�������ͷ�ι��е�ˮ��NH3+H2O?NH3?H2O?NH4++OH-��
��4������ʱ��ƿ��ѹǿ��������������ͣ���������ˮ�Ӵ�ʱ��������������ˮ��������ƿ��ѹǿѸ�ټ�С���γ���Ȫ��
�ʴ�Ϊ�����ӣ����֣�����ë���ȣ�����ƿ���ȣ����������������ͣ��ϳ����������ڵĿ�����������ˮ�Ӵ�����������Ȫ��
���������⿼�鰱�����Ʊ������ʣ���Ŀ�ѶȲ�����ע���γ���Ȫ��ԭ���Ͳ���������

��ϰ��ϵ�д�
�����Ŀ
 ����ѹǿ����������ʵ��ͻ�ѧʵ��������ҪӰ�죬��ȡ�����������Ȫʵ�飮
����ѹǿ����������ʵ��ͻ�ѧʵ��������ҪӰ�죬��ȡ�����������Ȫʵ�飮


