��Ŀ����
����Ŀ����������������������Ʒ��������̨������Ȧ����ʽ���У� ����ƿ ����ʽ�ζ��ܺͼ�ʽ�ζ��� ���ձ� �ݲ����� ��ͷ�ι� ����ƽ�������룩 ����ֽ ����Ͳ �����©����
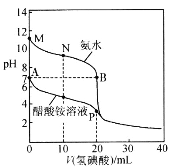
��������ҩƷ����A��NaOH���� ��B����NaOH��Һ��C��δ֪Ũ������ ��D������ˮ��E��̼������Һ
������������ѧ��ʵ�飬�ش��������⣺
��1������ʱ��Ӧѡ�õ�����������_________________________�����ţ���
��2������һ�����ʵ���Ũ�ȵ�NaOH��Һʱ����ȱ�ٵ�������__________��
��3��������к͵ζ�ʱ����ȱ�����Լ���____________________��
��4�������к͵ζ�ʱ���������ý�Ҫʢ����Һ������ϴ�����������е�______�����ţ����ף���ʽ�ζ��� �ң���ʽ�ζ��� ������ƿ
��5���ñ���NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬѡ�÷�̪��Ϊָʾ������ɲⶨ���ƫ�ߵ�ԭ�������______________��
A�����Ʊ���Һ��NaOH�л���Na2CO3����
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�����������ȷ
C��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ
D���ζ�ǰ�����ݣ��ζ���������ʧ��
II. �״���CH3OH����һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��1����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��ӦI��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1
CH3OH(g)+H2O(g) ��H1
��ӦII��CO(g)+2H2(g)![]() CH3OH(g) ��H2
CH3OH(g) ��H2
�ٷ�ӦI��ƽ�ⳣ������ʽΪK=________________________
���±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)
�¶� | 250�� | 300�� | 350�� |
K | 2.041 | 0.270 | 0.012 |
�ɱ��������ж���H2______0����S______0 (��������������������������)��
��ij�¶��£���2 mol CO��6 mol H2����2L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)=0.2 mol��L-1����CO��ת����Ϊ________��
��2����֪�ڳ��³�ѹ�£�ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ�����������ͼ��ʾ�ĵ��װ�ã�

��2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(g) ��H ��-1275.6 kJ��mol-1
��H2O(g)��H2O(l) ��H ��-44.0 kJ��mol-1
��д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ____________________��
��д����ͼ���װ���и�����Ӧʽ��________________________________��
���𰸡��٢ݢ�� ����ƿ ���ָʾ��(��̪��Һ�����) �� AD ![]() �� �� 80�� CH3OH(l)+
�� �� 80�� CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l)��H=-725.8kJ�Mmol CH3OH-6e-+8OH-=CO32-+6H2O
O2(g)=CO2(g)+2H2O(l)��H=-725.8kJ�Mmol CH3OH-6e-+8OH-=CO32-+6H2O
��������
��(1)�����ǽ����岻�����Һ������һ�ַ�������װ��������̨���ձ�����������©������������ֽ��ɣ�
(2)�������Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
(3)��������к͵ζ�����Ҫ���Լ���������
(4)�к͵ζ���Ҫ�õ���ʽ�ζ��ܡ���ʽ�ζ��ܺ���ƿ�����ʵ���ע����������жϣ�
(5)����c(��)=![]() �����ζ������в�����������ҺŨ�ȱ������ı�Һ���ƫС���ζ����ƫ�ͣ�����ƫ�ߣ��ݴ˽��н��
�����ζ������в�����������ҺŨ�ȱ������ı�Һ���ƫС���ζ����ƫ�ͣ�����ƫ�ߣ��ݴ˽��н��
II. (1)������ƽ�ⳣ��K=![]() ��д�����ɱ������ݣ�ƽ�ⳣ�����¶�����С��˵�������¶ȣ�ƽ�������ƶ�����Ϸ���ʽ������𣻢۴ﵽƽ����c(CO)=0.2molL-1�����Լ���ƽ��ʱCO�����ʵ������Ӷ������Ӧ��CO�����ʵ������ټ���CO��ת���ʣ�
��д�����ɱ������ݣ�ƽ�ⳣ�����¶�����С��˵�������¶ȣ�ƽ�������ƶ�����Ϸ���ʽ������𣻢۴ﵽƽ����c(CO)=0.2molL-1�����Լ���ƽ��ʱCO�����ʵ������Ӷ������Ӧ��CO�����ʵ������ټ���CO��ת���ʣ�
(2)�����ݸ�˹���ɼ��㣻�ڸ�ȼ�ϵ���У�ͨ��״��ĵ缫�Ǹ�����ͨ�������ĵ缫����������������������Ӧ���ݴ���д�缫��Ӧʽ��
��(1)�����ǽ����岻�����Һ������һ�ַ�������װ��������̨���ձ�����������©������������ֽ��ɣ���Ϊ�٢ݢ�⣬�ʴ�Ϊ���٢ݢ�⣻
(2)����һ�����ʵ���Ũ�ȵ�NaOH��Һ�IJ��������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ�(������Ͳ��ȡˮ�����ձ�)�����ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�����ƿ�У����ò�����������ϴ���ձ���������2��3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ���������������ƿ����ͷ�ιܣ��ʻ�ȱ������ƿ���ʴ�Ϊ������ƿ��
(3)������к͵ζ��Ĺ����У���Ҫ���Լ�����Һ����Һ�����ָʾ�����ʻ���Ҫ�������ָʾ��(��̪��Һ�����)���ʴ�Ϊ�����ָʾ��(��̪��Һ�����)��
(4)�к͵ζ���Ҫ�õ���ʽ�ζ��ܡ���ʽ�ζ��ܺ���ƿ��������ʽ�ζ��ܡ���ʽ�ζ�����Ҫ��ϴ����ƿ������ϴ��������ƫ�ߣ��ʴ�Ϊ������
(5)A�����Ʊ���Һ��NaOH�л���Na2CO3���ʣ��Է�̪Ϊָʾ��ʱ�����������������ƺ�Na2CO3���ĵ���ǰ�ߴ����Ա���Һ�����������л���Na2CO3����ʱ���V(��)ƫ����c(��)=![]() ����֪c(��)ƫ�ߣ���A��ȷ��B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ�����±�Һ���������ƫС����ⶨ���ƫ�ͣ���B����C��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ��������ȷ������Ӱ��ⶨ�������C����D���ζ�ǰ�����ݣ��ζ���������ʧ���������ı�Һ���ƫ�ⶨ���ƫ�ߣ���D��ȷ���ʴ�Ϊ��AD��
����֪c(��)ƫ�ߣ���A��ȷ��B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ�����±�Һ���������ƫС����ⶨ���ƫ�ͣ���B����C��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ��������ȷ������Ӱ��ⶨ�������C����D���ζ�ǰ�����ݣ��ζ���������ʧ���������ı�Һ���ƫ�ⶨ���ƫ�ߣ���D��ȷ���ʴ�Ϊ��AD��
II. (1)�ٷ�ӦI��CO2(g)+3H2(g)CH3OH(g)+H2O(g)����Ӧ��ƽ�ⳣ��K=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
���ɱ������ݣ�ƽ�ⳣ�����¶�����С��˵�������¶ȣ�ƽ�������ƶ�������ӦΪ���ȷ�Ӧ����H2��0����Ӧǰ�����������С���ؼ�С�ķ�Ӧ����S��0���ʴ�Ϊ����������
��ij�¶��£���2molCO��6molH2����2L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)=0.2molL-1��ƽ��ʱCO�����ʵ���=0.2molL-1 ��2L=0.4mol����Ӧ��COΪ2mol-0.4mol=1.6mol����CO��ת����=![]() ��100%=80%���ʴ�Ϊ��80%��
��100%=80%���ʴ�Ϊ��80%��
(2)�١���2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(g)��H=-1275.6 kJ�Mmol����H2O(g)=H2O(l)��H=-44.0 kJ�Mmol�����ݸ�˹���ɼ��㣬�ɸ�˹���ɣ���+����4�ã�2CH3OH(l)+3O2(g)= 2CO2(g)+4H2O(l)������H=-1275.6kJ�Mmol+(-44.0kJ/mol)��4=-1451.6kJ�Mmol�����Լ״�ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽΪ��CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l)��H=-725.8kJ�Mmol���ʴ�Ϊ��CH3OH(l)+
O2(g)=CO2(g)+2H2O(l)��H=-725.8kJ�Mmol���ʴ�Ϊ��CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l)��H=-725.8kJ�Mmol��
O2(g)=CO2(g)+2H2O(l)��H=-725.8kJ�Mmol��
�ڸ�ȼ�ϵ���У�ͨ��״��ĵ缫�Ǹ�����ͨ�������ĵ缫��������������ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��������ӦʽΪO2+4e-+2H2O=4OH-���ʴ�Ϊ��CH3OH-6e-+8OH-=CO32-+6H2O��