��Ŀ����
������500mL0.2mol/L��̼������Һ���ش��������⣺
��1��������Ҫ������______��������ƿ�ϱ��п��ߡ�______��______��
��2��ͨ�������֪��Ӧ��������ƽ��ȡ______g̼���ƣ�
��3�������IJ���˳��Ϊ������ţ�______
A������ƽ��㡡���� B��������㡡����C����С�ձ��м���̼���ơ�����D���������ձ�������
E�������̼����벢�������Ƶ�����λ�á����� F��������غС������� G����¼�������
��4���������ҩƷλ�÷ŵߵ����������ʱδ���ձ�������ƽƽ��ʱ��ʵ�ʳƵ�̼���Ƶ�������______g��
��5�����в���ʹ������ҺŨ��ƫ�ߵ���______��
A����ȡ28.6gNa2CO3?10H2O�������ơ������������� B������ʱ�������������
C��������ƿת����Һʱ��������Һ�彦������������ D��̼�����к��в���������
E��δϴ���ܽ�̼���Ƶ��ձ����������������������� F������ʱ�����ӿ̶���
G��С�ձ�ϴ����δ���T���������������������� H������ƿδ���T����������Һ��
����������������ƽ��500mL����ƿ���ձ�������������ͷ�ιܡ�ҩ�ȣ�
����ƿ�ϱ��п��ߡ��¶ȡ�������
�ʴ�Ϊ��������ƽ��500mL����ƿ���ձ�������������ͷ�ιܡ�ҩ�ף��¶ȣ�������
��2����̼���Ƶ����ʵ���Ϊ0.5L��0.2mol?L-1=0.1mol����̼���Ƶ�����Ϊ0.1mol��106g/mol=10.6g���ʴ�Ϊ��10.6��
��3�������IJ���˳��Ϊ��������㣬����ƽ��㣬�������ձ�����¼��������������̼����벢�������Ƶ�����λ�ã���С�ձ��м���̼���ƣ�������غУ�
�ʴ�Ϊ��BADGECF��
��4�������ҩƷλ�÷ŵߵ����������ʱδ���ձ�����ʵ�ʳƵ�̼���Ƶ�����10.0g-0.6g=9.4g���ʴ�Ϊ��9.4��
��5��A����̼���Ƶ����ʵ���Ϊ0.1mol������Na2CO3?10H2O���ƣ���ҪNa2CO3?10H2O������Ϊ0.1mol��286g/mol=28.6g����Ӱ�죬��A��ѡ��
B��������������룬�����ʵ��������������̼���Ƶ�ʵ����������������Һ��Ũ��ƫ�ߣ���Bѡ��
C��������ƿת����Һʱ��������Һ�彦������������ƿ��̼���Ƶ�ʵ��������С��������Һ��Ũ��ƫ�ͣ���C��ѡ��
D��̼�����к��в��������ʣ�������̼���Ƶ�ʵ��������С��������Һ��Ũ��ƫ�ͣ���D��ѡ��
E��δϴ���ܽ�̼���Ƶ��ձ����ձ�����մ��������̼���ƣ���������ƿ��̼���Ƶ�ʵ��������С��������Һ��Ũ��ƫ�ͣ���E��ѡ��
F������ʱ�����ӿ̶��ߣ�������Һ�����ƫ����ҺŨ��ƫ�ͣ���F��ѡ��
G��С�ձ�ϴ����δ���T����������������̼���Ƶ�ʵ��������С��������Һ��Ũ��ƫ�ͣ���G��ѡ��
H���������ˮ���ݣ�����ƿδ���T����������Һ����������ҺŨ����Ӱ�죬��H��ѡ��
��ѡB��
��������1������������Һ��ʵ���������ѡ����������������ƿ�ϱ��п��ߡ��¶ȡ�������
��2������n=cv����̼���Ƶ����ʵ������ٸ���m=nM��������̼���Ƶ�������
��3�����ݳ�����ʵ�������������ƽ��ʹ�ý��
��4����ƽƽ��ԭ��Ϊ��m�����̣�=m�����̣�+����������ݴ˼��㣻
��5���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
 �����жϣ�
�����жϣ����������⿼����һ�����ʵ���Ũ����Һ�����ƣ��Ƚϻ�����ע���c=
 ��������ԭ���������������ƹ��̣�
��������ԭ���������������ƹ��̣� 
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
A������ƿδ���T����������Һ B��������ƿת����Һʱ������Һ�彦��
C��δϴ���ܽ������ձ� D������ʱ�����ӿ̶���
������ij��ѧС����ʵ��������ͼ�ṩ�������Ʊ������顣
��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����=== NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
|
| �ܶ�/g��mL-1 | �е�/�� | �ܽ��� |
| ������ | 1.461 | 38 | ������ˮ |
| �Ҵ� | 0.789 | 78 | ������ˮ |
�ش��������⣺
��4����������������˳���ǣ�1��( )��( )��(4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
| A������ƿδ���T����������Һ | B��������ƿת����Һʱ������Һ�彦�� |
| C��δϴ���ܽ������ձ� | D������ʱ�����ӿ̶��� |

��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����="==" NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
| | �ܶ�/g��mL-1 | �е�/�� | �ܽ��� |
| ������ | 1.461 | 38 | ������ˮ |
| �Ҵ� | 0.789 | 78 | ������ˮ |
��4����������������˳���ǣ�1��( )��( )��( 4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
A������ƿδ���T����������Һ B��������ƿת����Һʱ������Һ�彦��
C��δϴ���ܽ������ձ� D������ʱ�����ӿ̶���
������ij��ѧС����ʵ��������ͼ�ṩ�������Ʊ������顣
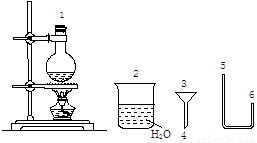
��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����=== NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
|
|
�ܶ�/g��mL-1 |
�е�/�� |
�ܽ��� |
|
������ |
1.461 |
38 |
������ˮ |
|
�Ҵ� |
0.789 |
78 |
������ˮ |
�ش��������⣺
��4����������������˳���ǣ�1��( )��( )��( 4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
