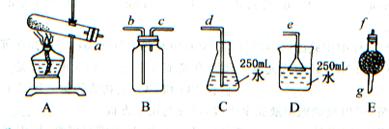
��Ŀ����
ȡwg�������ʣ��������Ȳ��ֽ⣩��NH4HCO3��ѡ����ͼ��ʾ��װ�ã���ȡһƿ����İ��������ఱ��ȫ����ˮ���ա��ش�
��1��ѡ�õ�װ���ǣ���A��B��C�����ش� ������ѡȡװ�õ���ȷ˳���ǣ�a��b��c������д���� �� �� �� �� �� ��
��2��Eװ������ʢҩƷ�������� ���������� ��
��3����ѡ��Bװ�ã�����ʱb�ڽ�������C�ڽ��� ����ԭ���� ��
��4�����ռ�������VmL����״��������ˮ��Ũ��Ϊa mol/L����̼����淋Ĵ���Ϊ
%����NH4HCO3ȫ���ֽ⣩��
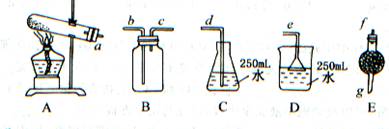
��1�� AEBD �� a �� f �� g �� c �� b �� e
��2����ʯ�� �� ����ˮ��CO2 ��
��3�� C�� �� �����ܶ�С�ڿ��� ����4����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
�ڳ����£�ij��ȤС��ģ�⡰�����Ƽ����ȡ̼���ƣ��������£�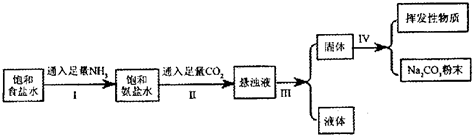
�ڳ����£��й����ʵ��ܽ��Ϊ��
��1���������е�ʵ���������Ϊ ��
��2������I�Ͳ���II�ܷ�Ӧ�����ӷ���ʽΪ ��
��3������I��II���ܵߵ���ԭ�� ��
��4���������õ�̼���Ʒ�ĩ�Ƿ���NaHCO3����ʵ�鷽���ǣ�д���������衢�����ۣ��� ��
��5��Ϊ�˲ⶨ����ȡ����Ĵ��ȣ���������ֻ��̼�����ƣ�����С���ʵ�鲽��Ϊ��
i��ʹ������װ����װʵ��װ�ã�����������ԣ�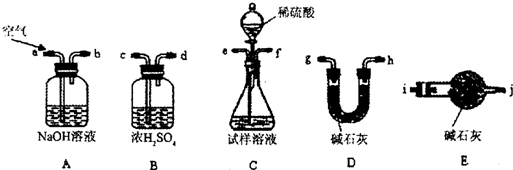
ii����ȡWg��Ʒ����Cװ�õ���ƿ�У�����������ˮ�ܽ�
iii������Dװ�õ�����ΪW1g
iv���ӷ�Һ©������ϡ���ᣬֱ�����ٲ�������Ϊֹ
v����a����������һ�����Ŀ������ٴγ���Dװ������ΪW2g��
vi���ظ�����v�IJ�����ֱ��Dװ�õ��������ٸı䣬�Ƶ�Dװ�õ�����ΪW3g��
��������ʵ��ش��������⣺
�ٵ�i����ʹ������װ�����ӵĽӿ�˳��Ϊ����b������e����f���� ��
�ڲ�����̼���Ƶ���������Ϊ�����ֿɲ��ػ��� ��

�ڳ����£��й����ʵ��ܽ��Ϊ��
| ���� | NH4Cl | NaHCO3 | Na2CO3 | NaCl |
| �ܽ��/g | 37.2 | 9.6 | 21.5 | 36.0 |
��2������I�Ͳ���II�ܷ�Ӧ�����ӷ���ʽΪ
��3������I��II���ܵߵ���ԭ��
��4���������õ�̼���Ʒ�ĩ�Ƿ���NaHCO3����ʵ�鷽���ǣ�д���������衢�����ۣ���
��5��Ϊ�˲ⶨ����ȡ����Ĵ��ȣ���������ֻ��̼�����ƣ�����С���ʵ�鲽��Ϊ��
i��ʹ������װ����װʵ��װ�ã�����������ԣ�

ii����ȡWg��Ʒ����Cװ�õ���ƿ�У�����������ˮ�ܽ�
iii������Dװ�õ�����ΪW1g
iv���ӷ�Һ©������ϡ���ᣬֱ�����ٲ�������Ϊֹ
v����a����������һ�����Ŀ������ٴγ���Dװ������ΪW2g��
vi���ظ�����v�IJ�����ֱ��Dװ�õ��������ٸı䣬�Ƶ�Dװ�õ�����ΪW3g��
��������ʵ��ش��������⣺
�ٵ�i����ʹ������װ�����ӵĽӿ�˳��Ϊ����b������e����f����
�ڲ�����̼���Ƶ���������Ϊ�����ֿɲ��ػ���