��Ŀ����
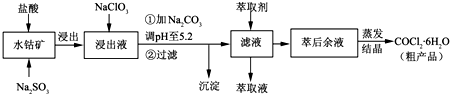
CoCl2��6H2O��һ������Ӫ��ǿ�������Ժ��ܷ��ϣ�������Fe��Al�����ʣ���ȡCoCl2��6H2O��һ���¹�����������ͼ

��֪��
���������ᷴӦ�Ļ�ѧ����ʽΪCo+2HCl=CoCl2+H2����
��CoCl2��6H2O���۵�Ϊ86�棬������ˮ�����ѵȣ��������ȶ�����������110~120��ʱ��ʧȥ�ᾧˮ����ж�����ˮ�Ȼ��ܡ�
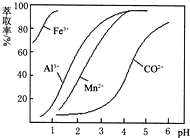
�۲���������������������ʽ����ʱ��Һ��pH���±�
���������ᷴӦ�Ļ�ѧ����ʽΪCo+2HCl=CoCl2+H2����
��CoCl2��6H2O���۵�Ϊ86�棬������ˮ�����ѵȣ��������ȶ�����������110~120��ʱ��ʧȥ�ᾧˮ����ж�����ˮ�Ȼ��ܡ�
�۲���������������������ʽ����ʱ��Һ��pH���±�

��ش��������⣺
(1)�������¹����У��á����ᡱ����ԭ�����С�����������Ļ��ᡱֱ���ܽ⺬�ܷ��ϣ�����Ҫ�ŵ���
___________________
(2)����̼���Ƶ���pH��a��a�ķ�Χ��_______________��
(3)����I����3������ʵ�������������____________���ˡ�
(4)�Ƶõ�CoCl2��6H2O���ѹ��ɵ�ԭ����______________________��
(5)Ϊ�ⶨ������CoCl2��6H2O�ĺ�����ijͬѧ��һ��������Ʒ����ˮ���������м���������AgNO3��Һ�����ˣ�����������ɺ������������ͨ�����㷢�ֲ�Ʒ��CoCl2��6H2O��������������100%����ԭ�������__________________��
(6)��ʵ�����У�Ϊ�˴�������Ʒ�л�ô�����CoCl2��6H2O�����õķ�����_____________��
(1)�������¹����У��á����ᡱ����ԭ�����С�����������Ļ��ᡱֱ���ܽ⺬�ܷ��ϣ�����Ҫ�ŵ���
___________________
(2)����̼���Ƶ���pH��a��a�ķ�Χ��_______________��
(3)����I����3������ʵ�������������____________���ˡ�
(4)�Ƶõ�CoCl2��6H2O���ѹ��ɵ�ԭ����______________________��
(5)Ϊ�ⶨ������CoCl2��6H2O�ĺ�����ijͬѧ��һ��������Ʒ����ˮ���������м���������AgNO3��Һ�����ˣ�����������ɺ������������ͨ�����㷢�ֲ�Ʒ��CoCl2��6H2O��������������100%����ԭ�������__________________��
(6)��ʵ�����У�Ϊ�˴�������Ʒ�л�ô�����CoCl2��6H2O�����õķ�����_____________��
(1)�����ж�������ŷţ���ֹ������Ⱦ����ֹ��Ʒ�л���������
(2)5.2��7.6
(3)����Ũ������ȴ�ᾧ
(4)���ͺ���¶ȣ���ֹ��Ʒ�ֽ�
(5)��Ʒ�к���NaCl���ʣ����ʱʧȥ�˲��ֽᾧˮ
(6)����Ʒ�������ѣ����˺�������
(2)5.2��7.6
(3)����Ũ������ȴ�ᾧ
(4)���ͺ���¶ȣ���ֹ��Ʒ�ֽ�
(5)��Ʒ�к���NaCl���ʣ����ʱʧȥ�˲��ֽᾧˮ
(6)����Ʒ�������ѣ����˺�������

��ϰ��ϵ�д�
�����Ŀ