ЬтФПФкШн
ЁОЬтФПЁПFeЁЂCoЁЂNiОљЮЊЕкЂјзхдЊЫиЃЌЫќУЧЕФЛЏКЯЮядкЩњВњЁЂЩњЛюжагазХЙуЗКЕФгІгУЁЃ
(1)ЛљЬЌCoдзгЕФМлЕчзгХХВМЪНЮЊ________ЃЌCo2ЃЋКЫЭт3dФмМЖЩЯга________ЖдГЩЖдЕчзгЁЃ
(2)Co3ЃЋЕФвЛжжХфРызг[Co(N3)(NH3)5]2ЃЋжаЃЌCo3ЃЋЕФХфЮЛЪ§ЪЧ________ЁЃ1 molХфРызгжаЫљКЌІвМќЕФЪ§ФПЮЊ____________ЃЌХфЮЛЬхN3ЃжааФдзгЕФдгЛЏРраЭЮЊ____________ЁЃ
(3)Co2ЃЋдкЫЎШмвКжавд[Co(H2O)6]2ЃЋДцдкЁЃЯђКЌCo2ЃЋЕФШмвКжаМгШыЙ§СПАБЫЎПЩЩњГЩИќЮШЖЈЕФ[Co(NH3)6]2ЃЋЃЌЦфдвђЪЧ__________________________ЁЃ
(4)ФГРЖЩЋОЇЬхжаЃЌFe2ЃЋЁЂFe3ЃЋЗжБ№еМОнСЂЗНЬхЛЅВЛЯрСкЕФЖЅЕуЃЌЖјСЂЗНЬхЕФУПЬѕРтЩЯОљгавЛИіCNЃЃЌKЃЋЮЛгкСЂЗНЬхЕФФГЧЁЕБЮЛжУЩЯЁЃОнДЫПЩжЊИУОЇЬхЕФЛЏбЇЪНЮЊ________ЃЌСЂЗНЬхжаFe2ЃЋМфСЌНгЦ№РДаЮГЩЕФПеМфЙЙаЭЪЧ________ЁЃ
(5)NiOЕФОЇАћНсЙЙШчЭММзЫљЪОЃЌЦфжадзгзјБъВЮЪ§AЮЊ(0,0,0)ЃЌBЮЊ(1,1,0)ЃЌдђCдзгзјБъВЮЪ§ЮЊ________ЁЃ
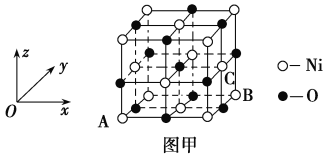
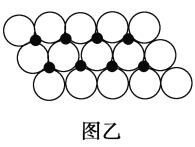
(6)вЛЖЈЮТЖШЯТЃЌNiOОЇЬхПЩвдздЗЂЕиЗжЩЂВЂаЮГЩЁАЕЅЗжзгВуЁБЃЌПЩвдШЯЮЊO2ЃЮЊУмжУЕЅВуХХСаЃЌNi2ЃЋЬюГфЦфжа(ШчЭМвв)ЃЌвбжЊO2ЃЕФАыОЖЮЊa pmЃЌУПЦНЗНУзУцЛ§ЩЯЗжЩЂЕФИУОЇЬхЕФжЪСПЮЊ_____g(гУКЌaЁЂNAЕФДњЪ§ЪНБэЪО)ЁЃ
ЁОД№АИЁП3d74s2 2 6 23NA sp NдЊЫиЕчИКадБШOдЊЫиЕчИКадаЁЃЌNдзгЬсЙЉЙТЕчзгЖдЕФЧуЯђИќДѓЃЌгыCo2ЃЋаЮГЩЕФХфЮЛМќИќЧП KFe2(CN)6 е§ЫФУцЬхаЮ (1ЃЌ1/2ЃЌ1/2) ![]()
ЁОНтЮіЁП
(1)ЛљЬЌCoдзгКЫЭтга27ИіЕчзгЃЌЦф3dЁЂ4sФмМЖЩЯЕФЕчзгЮЊЦфМлЕчзгЃЌCoдзгЪЇШЅ3ИіЕчзгЩњГЩCo3+ЃЌCo3+КЫЭт3dФмМЖЩЯга1ЖдГЩЖдЕчзгЃЛ
(2)Co3+ЕФвЛжжХфРызг[Co(N3)(NH3)5]2+жаЃЌCo3+ЕФХфЮЛЪ§ЪЧ6ЃЌ1ИіХфРызгжаЫљКЌІвМќЕФЪ§ФПЮЊ6+2+3ЁС5ЃЌХфЮЛЬхN3-жааФдзгМлВуЕчзгЖдИіЪ§=2+![]() =2ЃЌИљОнМлВуЕчзгЖдЛЅГтРэТлХаЖЯИУРызгдгЛЏРраЭЃЛ
=2ЃЌИљОнМлВуЕчзгЖдЛЅГтРэТлХаЖЯИУРызгдгЛЏРраЭЃЛ
(3)NдЊЫиЕчИКадБШOдЊЫиЕчИКадаЁЃЌNдзгЬсЙЉЙТЕчзгЖдЕФЧуЯђИќДѓЃЛ
(4)ИУОЇЬхжаFe2+ЁЂFe3+ЯрЕШЧвЕШгк4ЁС![]() =
=![]() ЁЂCN-ИіЪ§=12ЁС
ЁЂCN-ИіЪ§=12ЁС![]() =3ЃЌИљОнРызгОЇЬхГЪЕчжааджЊЃЌМиРызгИіЪ§=
=3ЃЌИљОнРызгОЇЬхГЪЕчжааджЊЃЌМиРызгИіЪ§= =0.5ЃЌСЂЗНЬхжаFe2+МфСЌНгЦ№РДаЮГЩЕФПеМфЙЙаЭЪЧе§ЫФУцЬхЃЛ
=0.5ЃЌСЂЗНЬхжаFe2+МфСЌНгЦ№РДаЮГЩЕФПеМфЙЙаЭЪЧе§ЫФУцЬхЃЛ
(5)NiOЕФОЇЬхНсЙЙШчЭММзЫљЪОЃЌЦфжаРызгзјБъВЮЪ§AЮЊ(0ЃЌ0ЃЌ0)ЃЌBЮЊ(1ЃЌ1ЃЌ0)ЃЌЫЕУїОЇАћРтГЄЮЊ1ЃЌCРызгЮЛгкОЇАћгвУцУцаФЩЯЃЌОрРыXжсЮЊ1ЁЂОрРыYжсЮЊ![]() ЁЂОрРыZжсЮЊ
ЁЂОрРыZжсЮЊ![]() ЃЛ
ЃЛ
(6)ИљОнЭМжЊЃЌУПИіNiдзгБЛ3ИіOдзгАќЮЇЁЂУПИіOдзгБЛ3ИіNiдзгАќЮЇЃЌШчЭМ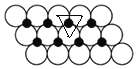 ЫљЪОЃЌЯрСкЕФ3ИідВжааФСЌЯпЮЊе§Ш§НЧаЮЃЌШ§НЧаЮЕФБпГЄЮЊ2apmЃЌУПИіШ§НЧаЮКЌгавЛИіNiдзгЃЌШ§НЧаЮЕФУцЛ§=[
ЫљЪОЃЌЯрСкЕФ3ИідВжааФСЌЯпЮЊе§Ш§НЧаЮЃЌШ§НЧаЮЕФБпГЄЮЊ2apmЃЌУПИіШ§НЧаЮКЌгавЛИіNiдзгЃЌШ§НЧаЮЕФУцЛ§=[![]() ЁС2aЁС2aЁСsin60ЁуЁС10-24]m2=
ЁС2aЁС2aЁСsin60ЁуЁС10-24]m2=![]() ЁС10-24a2m2ЃЌШчЭМ
ЁС10-24a2m2ЃЌШчЭМ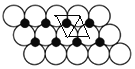 ЪЕМЪЩЯУПИіNiдзгБЛСНИіаЁШ§НЧаЮАќКЌаЁЦНааЫФБпаЮУцЛ§ЮЊ2
ЪЕМЪЩЯУПИіNiдзгБЛСНИіаЁШ§НЧаЮАќКЌаЁЦНааЫФБпаЮУцЛ§ЮЊ2![]() ЁС10-24a2m2ЃЌOдзгИіЪ§=
ЁС10-24a2m2ЃЌOдзгИіЪ§=![]() ЁС6=1ЃЌУПЦНЗНУзУцЛ§ЩЯЗжЩЂЕФИУОЇЬхЕФжЪСП=
ЁС6=1ЃЌУПЦНЗНУзУцЛ§ЩЯЗжЩЂЕФИУОЇЬхЕФжЪСП=![]() gЁЃ
gЁЃ
(1)ЛљЬЌCoдзгКЫЭтга27ИіЕчзгЃЌЦф3dЁЂ4sФмМЖЩЯЕФЕчзгЮЊЦфМлЕчзгЃЌCoдзгЪЇШЅ3ИіЕчзгЩњГЩCo3+ЃЌЦфКЫЭтМлЕчзгХХВМЪНЮЊ3d74s2ЃЌ3dФмМЖЩЯЛЙашвЊ3ИіЕчзгЬюТњЃЌCo3+КЫЭт3dФмМЖЩЯга2ЖдГЩЕчзгЃЛ
(2)Co3+ЕФвЛжжХфРызг[Co(N3)(NH3)5]2+жаЃЌCo3+ЕФХфЮЛЪ§ЪЧ6ЃЌ1ИіХфРызгжаЫљКЌІвМќЕФЪ§ФПЮЊ6+2+3ЁС5=23ЃЌ1molХфРызгжаЫљКЌІвМќЕФЪ§ФПЮЊ23NAЃЌХфЮЛЬхN3-жааФдзгМлВуЕчзгЖдИіЪ§=2+![]() =2ЃЌИљОнМлВуЕчзгЖдЛЅГтРэТлХаЖЯИУРызгдгЛЏРраЭЮЊspЃЛ
=2ЃЌИљОнМлВуЕчзгЖдЛЅГтРэТлХаЖЯИУРызгдгЛЏРраЭЮЊspЃЛ
(3)NдЊЫиЕчИКадБШOдЊЫиЕчИКадаЁЃЌNдзгЬсЙЉЙТЕчзгЖдЕФЧуЯђИќДѓЃЌгыCo2+аЮГЩЕФХфЮЛМќИќЧПЃЌЫљвдЯђКЌCo2+ЕФШмвКжаМгШыЙ§СПАБЫЎПЩЩњГЩИќЮШЖЈЕФ[Co(NH3)6]2+ЃЛ
(4)ИУОЇЬхжаFe2+ЁЂFe3+ЯрЕШЧвЕШгк4ЁС![]() =
=![]() ЁЂCN-ИіЪ§=12ЁС
ЁЂCN-ИіЪ§=12ЁС![]() =3ЃЌИљОнРызгОЇЬхГЪЕчжааджЊЃЌМиРызгИіЪ§=
=3ЃЌИљОнРызгОЇЬхГЪЕчжааджЊЃЌМиРызгИіЪ§=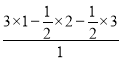 =0.5ЃЌдђИУОЇЬхжаМиРызгЁЂFeдзгЁЂCN-ИіЪ§жЎБШ=0.5ЃК(0.5ЁС2)ЃК3=1ЃК2ЃК6ЃЌЦфЛЏбЇЪНЮЊKFe2(CN)6ЃЌСЂЗНЬхжаFe2+МфСЌНгЦ№РДаЮГЩЕФПеМфЙЙаЭЪЧе§ЫФУцЬхЃЛ
=0.5ЃЌдђИУОЇЬхжаМиРызгЁЂFeдзгЁЂCN-ИіЪ§жЎБШ=0.5ЃК(0.5ЁС2)ЃК3=1ЃК2ЃК6ЃЌЦфЛЏбЇЪНЮЊKFe2(CN)6ЃЌСЂЗНЬхжаFe2+МфСЌНгЦ№РДаЮГЩЕФПеМфЙЙаЭЪЧе§ЫФУцЬхЃЛ
(5)NiOЕФОЇЬхНсЙЙШчЭММзЫљЪОЃЌЦфжаРызгзјБъВЮЪ§AЮЊ(0ЃЌ0ЃЌ0)ЃЌBЮЊ(1ЃЌ1ЃЌ0)ЃЌЫЕУїОЇАћРтГЄЮЊ1ЃЌCРызгЮЛгкОЇАћгвУцУцаФЩЯЃЌОрРыXжсЮЊ1ЁЂОрРыYжсЮЊ![]() ЁЂОрРыZжсЮЊ
ЁЂОрРыZжсЮЊ![]() ЃЌЫљвдCЕуВЮЪ§ЮЊ(1ЃЌ
ЃЌЫљвдCЕуВЮЪ§ЮЊ(1ЃЌ![]() ЃЌ
ЃЌ![]() )ЃЛ
)ЃЛ
(6)ИљОнЭМжЊЃЌУПИіNiдзгБЛ3ИіOдзгАќЮЇЁЂУПИіOдзгБЛ3ИіNiдзгАќЮЇЃЌШчЭМ ЫљЪОЃЌЯрСкЕФ3ИідВжааФСЌЯпЮЊе§Ш§НЧаЮЃЌШ§НЧаЮЕФБпГЄЮЊ2apmЃЌУПИіШ§НЧаЮКЌгавЛИіNiдзгЃЌШ§НЧаЮЕФУцЛ§=[
ЫљЪОЃЌЯрСкЕФ3ИідВжааФСЌЯпЮЊе§Ш§НЧаЮЃЌШ§НЧаЮЕФБпГЄЮЊ2apmЃЌУПИіШ§НЧаЮКЌгавЛИіNiдзгЃЌШ§НЧаЮЕФУцЛ§=[![]() ЁС2aЁС2aЁСsin60ЁуЁС10-24]m2=
ЁС2aЁС2aЁСsin60ЁуЁС10-24]m2=![]() ЁС10-24a2m2ЃЌШчЭМ
ЁС10-24a2m2ЃЌШчЭМ ЪЕМЪЩЯУПИіNiдзгБЛСНИіаЁШ§НЧаЮАќКЌаЁЦНааЫФБпаЮУцЛ§ЮЊ2
ЪЕМЪЩЯУПИіNiдзгБЛСНИіаЁШ§НЧаЮАќКЌаЁЦНааЫФБпаЮУцЛ§ЮЊ2![]() ЁС10-24a2m2ЃЌOдзгИіЪ§=
ЁС10-24a2m2ЃЌOдзгИіЪ§=![]() ЁС6=1ЃЌУПЦНЗНУзУцЛ§ЩЯЗжЩЂЕФИУОЇЬхЕФжЪСП=
ЁС6=1ЃЌУПЦНЗНУзУцЛ§ЩЯЗжЩЂЕФИУОЇЬхЕФжЪСП=![]() g=
g=![]() g=
g=![]() gЁЃ
gЁЃ

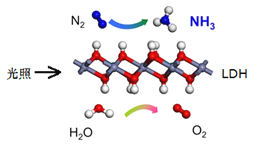
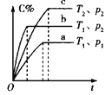
ЁОЬтФПЁПШчЭМЪЧЪЕбщЪвжЦБИТШЦјВЂНјаавЛЯЕСаЯрЙиЪЕбщЕФзАжУЃЈМаГжМАМгШШвЧЦївбТдЃЉЃЎ
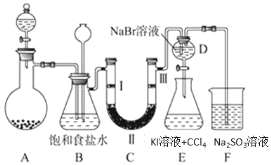
ЃЈ1ЃЉзАжУCЕФЪЕбщФПЕФЪЧбщжЄТШЦјЪЧЗёОпгаЦЏАзадЃЌЮЊДЫCжаЂёЁЂЂђЁЂЂѓвРДЮЗХ______
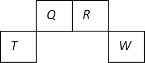
a | b | c | d | |
Ђё | ИЩдяЕФгаЩЋВМЬѕ | ИЩдяЕФгаЩЋВМЬѕ | ЪЊШѓЕФгаЩЋВМЬѕ | ЪЊШѓЕФгаЩЋВМЬѕ |
Ђђ | МюЪЏЛв | ЮоЫЎТШЛЏИЦ | ХЈСђЫс | ЮоЫЎТШЛЏИЦ |
Ђѓ | ЪЊШѓЕФгаЩЋВМЬѕ | ЪЊШѓЕФгаЩЋВМЬѕ | ИЩдяЕФгаЩЋВМЬѕ | ИЩдяЕФгаЩЋВМЬѕ |
ЃЈ2ЃЉЩшМЦзАжУDЁЂEЕФФПЕФЪЧБШНЯТШЁЂфхЁЂЕтЕЅжЪЕФбѕЛЏадЧПШѕЁЃЕБЯђDжаЛКЛКЭЈШывЛЖЈСПЕФТШЦјЪБЃЌПЩвдПДЕНЮоЩЋШмвКж№НЅБфЮЊГШЛЦЩЋЃЌДђПЊЛюШћЃЌНЋзАжУDжаЩйСПШмвКМгШызАжУEжаЃЌеёЕДЃЌЙлВьЕНЕФЯжЯѓЪЧЯТВуШмвКГЪзЯЩЋЃЌдђжЄУїСЫфхЕЅжЪЕФбѕЛЏадЧПгкЕтЕЅжЪЃЌЕЋЪЧгаЭЌбЇЖдИУНсТлЬсГівьвщЃЌПЩФмРэгЩЪЧ___________ЁЃ
ЃЈ3ЃЉЩеБFжаЕФ бЧСђЫсФЦШмвКгУРДЮќЪеЮВЦјЃЌЩшМЦЪЕбщЗНАИбщжЄЮВЦјЮќЪеКѓШмвКжаКЌга SO42-_____ЁЃ