��Ŀ����
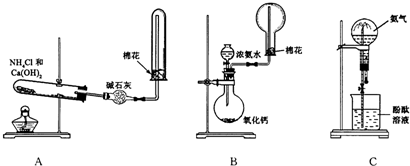
(10��)ij����С������ȡ�������ƺ��Ȼ��ƵĻ����Һ��Ϊ��ߴ������ƺ���������ͼ��ʾװ�ã�ͼ��ƿ��ʢ����ʳ��ˮ��ƿ��ʢŨ���ᣬ��Һ©��A��ʢŨ���ᣮ(��������ʾ��Cl2��NaOH�ڲ�ͬ�¶��£����ﲻͬ���ڽϸ��¶���������NaClO3)��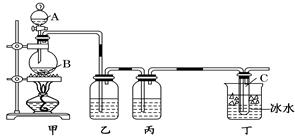
�Իش�
(1)��ƿB��ʢ�����������Թ�C��ʢ�������� .
(2)��ͬѧ��Ϊ����ʡȥijЩװ�ã�����Ϊ�������ܷ�ʡȥ��װ�ã���������(��ܡ����ܡ�)��������
(3)��ͬѧ��Ϊ���������ijЩװ�ã�����Ϊ������ (���Ҫ������Ҫ��)���������Ϊ��Ҫ����ָ����װ�õ�����
(4)��װ���б�ˮ�������� ��
(1)MnO2����1�֣� NaOH��Һ��1�֣�
(2)���ܡ���1�֣�HCl�������C�У�����NaOH������NaClO�ĺ�������2�֣�
(3)��Ҫ����1�֣�Ӧ����β������װ�ã���ֹCl2��Ⱦ������2�֣���
(4)��ֹCl2��NaOH��Һ���¶Ƚϸ�ʱ������������Ӧ��2�֣�
����