��Ŀ����
��״���£�1.68L��ɫ��ȼ������������������ȫȼ�ա���������ͨ�������ij���ʯ��ˮ���õ��İ�ɫ��������Ϊ15.0g������������ʯ������ȼ�ղ������9. 3g��
(1)����ȼ�ղ�����ˮ������Ϊ___________g��
(2)�������ǵ�һ���壬����ȫȼ�շų���������____________��
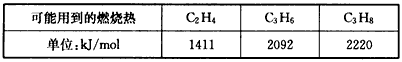
(3)��ԭ���������ֵ����ʵ����������������һ����������һ�������ȼ����Ϊ287kJ/mol�����������ȫȼ�չ�����94kJ�������ȼ�յ��Ȼ�ѧ����ʽΪ____________________��
(2)�������ǵ�һ���壬����ȫȼ�շų���������____________��
(3)��ԭ���������ֵ����ʵ����������������һ����������һ�������ȼ����Ϊ287kJ/mol�����������ȫȼ�չ�����94kJ�������ȼ�յ��Ȼ�ѧ����ʽΪ____________________��
(1)2.7
(2)105.8kJ
(3)C3H8(g)+5O2(g)=3CO2(g)+4H2O(l) ��H=-2220kJ/mol
(2)105.8kJ
(3)C3H8(g)+5O2(g)=3CO2(g)+4H2O(l) ��H=-2220kJ/mol

��ϰ��ϵ�д�
�����Ŀ