��Ŀ����
����Ԫ��A��B��C��D����ԭ�ӽṹ�������Ϣ���±���
��ش��������⣺
��1��A��ԭ�ӽṹʾ��ͼ�� ��DԪ��λ�����ڱ��� ���� �壮
��2������B��A�γɵ��ڶ�����У�ֻҪBԭ�ӵijɼ�������� ������ĸ��ţ������������е�ԭ�Ӿ��п�����ͬһ��ƽ���ڣ�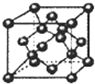
A��Sp3 ��Sp2 �ӻ���� B��sp3 ��sp �ӻ���� C��sp ��sp2 �ӻ����
��3����ͼ����B�����һ�������к��� ��Bԭ�ӣ�
��4���Ʊ�C2A4�ķ������ô������ƣ�NaClO����Һ����������CA3��
��CA3������ ������ԡ��Ǽ��ԡ������ӣ�
��д���Ʊ�C2A4���ܷ�Ӧ����ʽ
��5��DC13��������ˮ��Һ�����ԣ���ԭ���ǣ������ӷ���ʽ��ʾ���� ��
| Ԫ�� | �����Ϣ |
| A | Aԭ�ӵĺ�����������������̬���Ӳ��� |
| B | Bԭ�ӵ������������Ǵ�����������2�� |
| C | C�Ļ�̬ԭ��L���Ӳ�����3��δ�ɶԵ��� |
| D | D����Χ���Ӳ��Ų�Ϊ��n+1��d3n��n+2��sn |
��1��A��ԭ�ӽṹʾ��ͼ��
��2������B��A�γɵ��ڶ�����У�ֻҪBԭ�ӵijɼ��������

A��Sp3 ��Sp2 �ӻ���� B��sp3 ��sp �ӻ���� C��sp ��sp2 �ӻ����
��3����ͼ����B�����һ�������к���
��4���Ʊ�C2A4�ķ������ô������ƣ�NaClO����Һ����������CA3��
��CA3������
��д���Ʊ�C2A4���ܷ�Ӧ����ʽ
��5��DC13��������ˮ��Һ�����ԣ���ԭ���ǣ������ӷ���ʽ��ʾ����
������Aԭ�ӵĺ�����������������̬���Ӳ�������AΪHԪ�أ�Bԭ�ӵ������������Ǵ�����������2������Bԭ�Ӻ�����2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�C�Ļ�̬ԭ��L���Ӳ�����3��δ�ɶԵ��ӣ���Cԭ�Ӻ�������Ų�ʽΪ1s22s22p3����CΪNԪ�أ�D����Χ���Ӳ��Ų�Ϊ��n+1��d3n��n+2��sn������d�ܼ�������Ӳ�����Ϊ3��s�ܼ��������2�����ӣ���n=2����D��Χ���Ӳ��Ų�Ϊ3d64s2����DΪFe���ݴ˽��
����⣺Aԭ�ӵĺ�����������������̬���Ӳ�������AΪHԪ�أ�Bԭ�ӵ������������Ǵ�����������2������Bԭ�Ӻ�����2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�C�Ļ�̬ԭ��L���Ӳ�����3��δ�ɶԵ��ӣ���Cԭ�Ӻ�������Ų�ʽΪ1s22s22p3����CΪNԪ�أ�D����Χ���Ӳ��Ų�Ϊ��n+1��d3n��n+2��sn������d�ܼ�������Ӳ�����Ϊ3��s�ܼ��������2�����ӣ���n=2����D��Χ���Ӳ��Ų�Ϊ3d64s2����DΪFe��
��1��AΪHԪ�أ�ԭ�ӽṹʾ��ͼΪ ��DΪFeԪ�أ�λ�����ڱ��������ڢ��壬
��DΪFeԪ�أ�λ�����ڱ��������ڢ��壬
�ʴ�Ϊ�� ���ģ�����
���ģ�����
��2������C��H�γɵ��ڶ�����У�̼ԭ��ֻҪ�γ�C=C˫����C��C���������������е�ԭ�Ӿ��п�����ͬһ��ƽ���ڣ��γ�C=C˫����Cԭ�Ӽ۲���Ӷ���=1+2=3��Cԭ�Ӳ�ȡSp2�ӻ����γ�C��C������Cԭ�Ӽ۲���Ӷ�ˮ=1+1=2����ȡsp�ӻ���
�ʴ�Ϊ��C��
��3����ͼB���徧���к���4��Bԭ�ӣ�������BΪһ�������ṩ
��������BΪ1�������ṩ
���ʾ����к���Bԭ����ĿΪ4+8��
+6��
=8��
�ʴ�Ϊ��8��
��4���Ʊ�N2H4�ķ������ô������ƣ�NaClO����Һ����������NH3��
��NH3����Ϊ�����ͣ�������ɲ��غϣ�Ϊ���Է��ӣ��ʴ�Ϊ�����ԣ���
�ڷ�Ӧ��NԪ�ػ��ϼ�����������Ԫ�ػ��ϼ۽��ͣ�Ӧ����NaCl������ԭ���غ㻹��ˮ���ɣ����Ʊ�N2H4���ܷ�Ӧ����ʽΪ��NaClO+2NH3=N2H4+NaCl+H2O��
�ʴ�Ϊ��NaClO+2NH3=N2H4+NaCl+H2O��
��5��FeC13ˮ��Һ�У�Fe3+ˮ��Fe3++3H2O?Fe��OH��3+3OH-���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ��ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3OH-��
��1��AΪHԪ�أ�ԭ�ӽṹʾ��ͼΪ
 ��DΪFeԪ�أ�λ�����ڱ��������ڢ��壬
��DΪFeԪ�أ�λ�����ڱ��������ڢ��壬�ʴ�Ϊ��
 ���ģ�����
���ģ�������2������C��H�γɵ��ڶ�����У�̼ԭ��ֻҪ�γ�C=C˫����C��C���������������е�ԭ�Ӿ��п�����ͬһ��ƽ���ڣ��γ�C=C˫����Cԭ�Ӽ۲���Ӷ���=1+2=3��Cԭ�Ӳ�ȡSp2�ӻ����γ�C��C������Cԭ�Ӽ۲���Ӷ�ˮ=1+1=2����ȡsp�ӻ���
�ʴ�Ϊ��C��
��3����ͼB���徧���к���4��Bԭ�ӣ�������BΪһ�������ṩ
| 1 |
| 2 |
| 1 |
| 8 |
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ��8��
��4���Ʊ�N2H4�ķ������ô������ƣ�NaClO����Һ����������NH3��
��NH3����Ϊ�����ͣ�������ɲ��غϣ�Ϊ���Է��ӣ��ʴ�Ϊ�����ԣ���
�ڷ�Ӧ��NԪ�ػ��ϼ�����������Ԫ�ػ��ϼ۽��ͣ�Ӧ����NaCl������ԭ���غ㻹��ˮ���ɣ����Ʊ�N2H4���ܷ�Ӧ����ʽΪ��NaClO+2NH3=N2H4+NaCl+H2O��
�ʴ�Ϊ��NaClO+2NH3=N2H4+NaCl+H2O��
��5��FeC13ˮ��Һ�У�Fe3+ˮ��Fe3++3H2O?Fe��OH��3+3OH-���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ��ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3OH-��
������������Ԫ���ƶ�Ϊ���壬�����������Ų����ɡ����û�ѧ����ӻ����ۡ������ṹ�����ӽṹ�����ʡ�������ԭ��Ӧ������ˮ��ȣ���Ŀ�ۺ��Խϴ��ۺϿ���֪ʶǨ�������������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ