��Ŀ����
��ú��Ϊȼ�Ͽ�ͨ����������;��:
;����C(s)+O2(g) CO2(g)����H1<0��
CO2(g)����H1<0��
;�������Ƴ�ˮú��:
C(s)+H2O(g) CO(g)+H2(g)����H2>0��
CO(g)+H2(g)����H2>0��
��ȼ��ˮú��:
2CO(g)+O2(g) 2CO2(g)����H3<0��
2CO2(g)����H3<0��
2H2(g)+O2(g) 2H2O(g)����H4<0��
2H2O(g)����H4<0��
��ش���������:
(1);����ų���������������������(����ڡ������ڡ���С�ڡ�);����ų���������
(2)��H1����H2����H3����H4����ѧ��ϵʽ������
(3)��֪:��C(s)+O2(g) CO2(g)����H1="-393.5" kJ��mol-1
CO2(g)����H1="-393.5" kJ��mol-1
��2CO(g)+O2(g) 2CO2(g)����H2="-566" kJ��mol-1
2CO2(g)����H2="-566" kJ��mol-1
��TiO2(s)+2Cl2(g) TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
��TiO2(s)+2Cl2(g)+2C(s) TiCl4(s)+2CO(g)�Ħ�H=����������
TiCl4(s)+2CO(g)�Ħ�H=����������
(4)��֪���и����Ȼ�ѧ����ʽ
��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
��3Fe2O3(s)+CO(g) 2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
��Fe3O4(s)+CO(g) 3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
��д��FeO(s)��CO(g)��ԭ��Fe��CO2(g)���Ȼ�ѧ����ʽ��______________________��
;����C(s)+O2(g)
 CO2(g)����H1<0��
CO2(g)����H1<0��;�������Ƴ�ˮú��:
C(s)+H2O(g)
 CO(g)+H2(g)����H2>0��
CO(g)+H2(g)����H2>0����ȼ��ˮú��:
2CO(g)+O2(g)
 2CO2(g)����H3<0��
2CO2(g)����H3<0��2H2(g)+O2(g)
 2H2O(g)����H4<0��
2H2O(g)����H4<0����ش���������:
(1);����ų���������������������(����ڡ������ڡ���С�ڡ�);����ų���������
(2)��H1����H2����H3����H4����ѧ��ϵʽ������
(3)��֪:��C(s)+O2(g)
 CO2(g)����H1="-393.5" kJ��mol-1
CO2(g)����H1="-393.5" kJ��mol-1��2CO(g)+O2(g)
 2CO2(g)����H2="-566" kJ��mol-1
2CO2(g)����H2="-566" kJ��mol-1��TiO2(s)+2Cl2(g)
 TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
TiCl4(s)+O2(g)����H3="+141" kJ��mol-1��TiO2(s)+2Cl2(g)+2C(s)
 TiCl4(s)+2CO(g)�Ħ�H=����������
TiCl4(s)+2CO(g)�Ħ�H=���������� (4)��֪���и����Ȼ�ѧ����ʽ
��Fe2O3(s)+3CO(g)
 2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1��3Fe2O3(s)+CO(g)
 2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1��Fe3O4(s)+CO(g)
 3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
3FeO(s)+CO2(g)����H3="+640" kJ��mol-1��д��FeO(s)��CO(g)��ԭ��Fe��CO2(g)���Ȼ�ѧ����ʽ��______________________��
(1)���ڡ�
(2)��H1=��H2+(��H3+��H4)/2
(3)-80 kJ��mol-1
(4)FeO(s)+CO(g) Fe(s)+CO2(g)����H="-218" kJ��mol-1
Fe(s)+CO2(g)����H="-218" kJ��mol-1
(2)��H1=��H2+(��H3+��H4)/2
(3)-80 kJ��mol-1
(4)FeO(s)+CO(g)
 Fe(s)+CO2(g)����H="-218" kJ��mol-1
Fe(s)+CO2(g)����H="-218" kJ��mol-1(1)���ݸ�˹���ɵĺ���֪,;����ų�����������;����ų���������
(2)�ɸ�˹����:��=�æ�H1=��H2+��H3+��H4��
(3)�ɢ�+2����-�ڵ�����ʽ,����H=��H3+2��H1-��H2="+141" kJ��mol-1+2��(-393.5 kJ��mol-1)-(-566 kJ��mol-1)="-80" kJ��mol-1��
(4)������:��FeO(s)+CO(g) Fe(s)+CO2(g)����H
Fe(s)+CO2(g)����H
���=�� ��H="=-218" kJ��mol-1
��FeO(s)+CO(g) Fe(s)+CO2(g)����H="-218" kJ��mol-1
Fe(s)+CO2(g)����H="-218" kJ��mol-1
(2)�ɸ�˹����:��=�æ�H1=��H2+��H3+��H4��
(3)�ɢ�+2����-�ڵ�����ʽ,����H=��H3+2��H1-��H2="+141" kJ��mol-1+2��(-393.5 kJ��mol-1)-(-566 kJ��mol-1)="-80" kJ��mol-1��
(4)������:��FeO(s)+CO(g)
 Fe(s)+CO2(g)����H
Fe(s)+CO2(g)����H���=�� ��H="=-218" kJ��mol-1
��FeO(s)+CO(g)
 Fe(s)+CO2(g)����H="-218" kJ��mol-1
Fe(s)+CO2(g)����H="-218" kJ��mol-1
��ϰ��ϵ�д�
�����Ŀ
 H2(g)+
H2(g)+  2NO2(g) ��H ����ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g) ��H ����ϵ�У�n(NO)��ʱ��ı仯�����

 CO(g)��2H2(g)��H2�����ʵ�����ʱ��仯��������ͼ��ʾ��
CO(g)��2H2(g)��H2�����ʵ�����ʱ��仯��������ͼ��ʾ��
 CH3OH(g)��ƽ�ⳣ��K��__________����ͬ�¶��£�����ʼʱ����CH3OH(g)�����ʵ�����ԭ����2������__________(�����)��ԭ����2����
CH3OH(g)��ƽ�ⳣ��K��__________����ͬ�¶��£�����ʼʱ����CH3OH(g)�����ʵ�����ԭ����2������__________(�����)��ԭ����2���� 2NH3(g) ��H=��92��4kJ/mol
2NH3(g) ��H=��92��4kJ/mol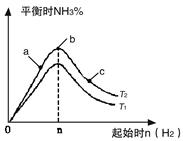
 4NO2(g)��O2(g) ��H��0�±�Ϊ��Ӧ��T1�¶��µIJ���ʵ������
4NO2(g)��O2(g) ��H��0�±�Ϊ��Ӧ��T1�¶��µIJ���ʵ������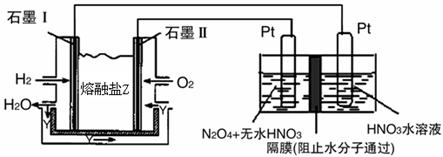
 CO2(g)+2H2O(l)����H1=-Q1
CO2(g)+2H2O(l)����H1=-Q1 2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����(
2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����( )����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
)����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
 CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H
 ��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ ��
��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ ��