��Ŀ����
�ҹ�����ר�Һ�°�ġ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�������NaHCO3��NaCl��NH4Cl�������ܽ�ȵIJ��죬��ʳ�Ρ�������������̼��Ϊԭ�����Ƶ�NaHCO3�����������������������ʵ������ģ�⡰�����Ƽ����ȡNaHCO3��ʵ�鲽�裺��һ�������Ӻ�װ�ã����������ԣ���������װ��ҩƷ��
�ڶ���������һװ�÷�����Ӧ��ֱ�����������岻������C���ܽ�ʱ����ͨ����һװ���в��������壬Ƭ�̺�C�г��ֹ��塣������C��ͨ���������壬ֱ�������й��������
������������C�����õĻ����õ�NaHCO3���塣
���IJ�������Һ�м���������NaCl��ĩ����NH4Cl��������������
������������⣺
��1��װ�õ�����˳���ǣ���a���ӣ� �� �� ���ӣ� ������b���ӣ� ��

��2��A�г�ѡ�õĹ��巴Ӧ��Ϊ____________��D��Ӧѡ�õ�Һ��Ϊ____________��B�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________________________��
��3���ڶ������б�������_______________װ���ȷ�����Ӧ��
��4��C�������θ���ܶ�����ֱ���ܣ���������____________________________________��C�й��ƿ�ڲ���������ܻ�ѧ����ʽΪ______________________________________��
��5�����IJ��з����NH4Cl����IJ�����___________________�������õ�NH4Cl�����г�����������NaCl��NaHCO3��Լռ5%��8%���������һ����ʵ��֤�����ù���ijɷִ���NH4Cl����Ҫд������������______________________________________��
��1��f e d c
��2����״ʯ��ʯ ����NaHCO3��Һ CaO+NH3��H2O====Ca��OH��2+NH3�������������𰸾��ɣ�
��3��B
��4�������� CO2+NH3+NaCl+H2O====NaHCO3��+NH4Cl
��5������ ȡ������������Թ��У����ȣ��������ʧ�����Թܿ����н϶�Ĺ������ᣨ���������𰸾��ɣ�
�������������֪�����Ƶ�NH3��CO2�������壬Ȼ������Ⱥ�ͨ�뱥��ʳ��ˮ�У����ɷ�����Ӧ������̼�����Ƴ���������ͨ������̼���壬���ڱ���ʳ��ˮ�е��ܽ�Ȳ�����ͨ���������ɵ�̼�����Ƶ����١���Ӧ��ͨ��������ͨ������̼��
�������������ʯ�ƵõĶ�����̼�����У������Ȼ������壬������̼�����Ʒ�Ӧ��Ӱ��̼�����ƵIJ���������Ӧ��ȥ������̼�л��е��Ȼ��⣬Ҳ����Ӧ��������ͨ�뱥��̼��������Һ�С�
���ڰ�����������ˮ��Ϊ��������Ӧ�����ܿڸսӴ�ˮ�棬�����ø���ܽ�һ������Ԥ����
�Ȼ�淋�ʵ��֤�������ü��ȷֽⷨ����ʣ�����Ķ��٣��Ӷ�˵���京���Ķ��١�


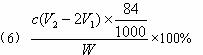


 H4C1������
H4C1������ �ܽ�ȵIJ��죬��ʳ
�ܽ�ȵIJ��죬��ʳ �Ρ�������������̼��Ϊԭ���Ƶ�NaHCO3�������������������A��B��C��D�ĸ�װ�ÿ���װ��ʵ����ģ�� �������Ƽ����ȡNaHCO3��ʵ��װ�á�װ���зֱ�ʢ�������Լ���B��ϡ���C�����ᡢ̼
�Ρ�������������̼��Ϊԭ���Ƶ�NaHCO3�������������������A��B��C��D�ĸ�װ�ÿ���װ��ʵ����ģ�� �������Ƽ����ȡNaHCO3��ʵ��װ�á�װ���зֱ�ʢ�������Լ���B��ϡ���C�����ᡢ̼ ��ƣ�D�������ı���ʳ��ˮ��ˮ
��ƣ�D�������ı���ʳ��ˮ��ˮ

 ��Դ:Z|xx|k.Com]
��Դ:Z|xx|k.Com] 5������ƿ�еIJ�����˺����õ�ĸҺ�к��� ���Ի�ѧʽ��ʾ���������Ȼ��⣬������ ������ʹNaCl��Һѭ��ʹ�ã�ͬʱ�ɻ���NH4C1��
5������ƿ�еIJ�����˺����õ�ĸҺ�к��� ���Ի�ѧʽ��ʾ���������Ȼ��⣬������ ������ʹNaCl��Һѭ��ʹ�ã�ͬʱ�ɻ���NH4C1��