题目内容
已知:Fe2O3(s) +  C(s) =
C(s) =  CO2(g) + 2 Fe(s) ΔΗ="234.1" kJ·mol-1
CO2(g) + 2 Fe(s) ΔΗ="234.1" kJ·mol-1
C(s) + O2(g) = CO2(g) ΔΗ=-393.5 kJ·mol-1
则2 Fe(s)+ O2(g) = Fe2O3(s) 的ΔΗ是
O2(g) = Fe2O3(s) 的ΔΗ是
 C(s) =
C(s) =  CO2(g) + 2 Fe(s) ΔΗ="234.1" kJ·mol-1
CO2(g) + 2 Fe(s) ΔΗ="234.1" kJ·mol-1C(s) + O2(g) = CO2(g) ΔΗ=-393.5 kJ·mol-1
则2 Fe(s)+
 O2(g) = Fe2O3(s) 的ΔΗ是
O2(g) = Fe2O3(s) 的ΔΗ是| A.-824.4 kJ·mol-1 | B.-627.6 kJ·mol-1 |
| C.-744.7 kJ·mol-1 | D.-169.4 kJ·mol-1 |
A
 (2)=(1)就可得2 Fe(s)+
(2)=(1)就可得2 Fe(s)+  O2(g) = Fe2O3(s),则ΔΗ=
O2(g) = Fe2O3(s),则ΔΗ= ΔΗ2-ΔΗ1=-824.4 kJ·mol-1。
ΔΗ2-ΔΗ1=-824.4 kJ·mol-1。
练习册系列答案
相关题目

 .
. 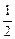 O2(g)===H2O(l) ΔH=-285.8 kJ·mol-1
O2(g)===H2O(l) ΔH=-285.8 kJ·mol-1 (1)合成氨反应反应N2(g)+3H2(g)
(1)合成氨反应反应N2(g)+3H2(g) 2NH3(g),若在恒温、恒压条件下向平衡体系中通入氩气,平衡 移动(填“向左”“向右”或“不”);,使用催化剂 反应的ΔH(填“增大”“减小”或“不改变”)。
2NH3(g),若在恒温、恒压条件下向平衡体系中通入氩气,平衡 移动(填“向左”“向右”或“不”);,使用催化剂 反应的ΔH(填“增大”“减小”或“不改变”)。 H1=" 1175.7" kJ·mol-1
H1=" 1175.7" kJ·mol-1 H3="482.2" kJ·mol-1
H3="482.2" kJ·mol-1 H2O+NH3↑
H2O+NH3↑