��Ŀ����
ij������Һ��ֻ����NH4+��Cl-��H+��OH-4�����ӣ�����˵������ȷ����
A������pH=3��HCl��pH=11��NH3��H2O��Һ�������϶���
B������Һ�����Ӽ�һ�����㣺c��NH4+��+c��H+��= c��OH-��+c��Cl-��
C����������NH3��H2O����Һ������Ũ�ȿ���Ϊ��c��NH4+��>c��C1-��>c��OH-��>c��H+��
D������Һ�����ɵ����ʵ���Ũ�ȡ��������HCl��Һ��NH3��H2O��Һ��϶���
���𰸡�
A
����������

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ
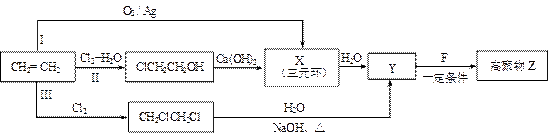
 ��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
 ����ˮ��ΪM��N b��һ��������M����ת��ΪN
����ˮ��ΪM��N b��һ��������M����ת��ΪN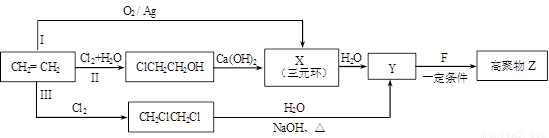
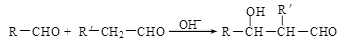 ��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��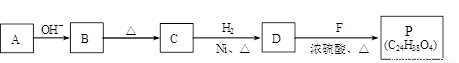

 ��R��R��������������ԭ�ӣ���
��R��R��������������ԭ�ӣ���