��Ŀ����
±�����ڼ��Դ���Һ���ܷ�����ȥ��Ӧ������
�÷�ӦҲ�ɱ�ʾΪ��
�����ǰ˸��л��������ת����ϵ��

������������⣺
��1������ϵͳ��������������A��������__________________��
��2��������ͼ�У�����________________��Ӧ������________________��Ӧ�����Ӧ���
��3��������E����Ҫ�Ĺ�ҵԭ�ϣ�д����D����E�Ļ�ѧ����ʽ��________________��
��4��C2�Ľṹ��ʽ��________________��
F1�Ľṹ��ʽ��________________��
F1��F2��Ϊ________________��
��5�������˸��������У����ڶ�ϩ������_____________����ϩ����ͨʽ��______________��
��1��2��3-��������
��2��ȡ�� �ӳ�
��3��

��4����CH3��2C=C��CH3��2 ![]() ͬ���칹��
ͬ���칹��
��5��E CnH2n-2
�����������ǰ���ʱȽϼ��ڣ�1���ʿ����л�����������ڣ�2���ʿ����л���Ӧ�Ļ������ͣ��ڣ�3������4�������������йط�Ӧ��������ȷ�����ʵĽṹ����B�Ľṹ����Ӧ������֪C1��C2����Ϊ��CH3��2CHC��CH3��=CH2��CH3��2C=C��CH3��2������E�ɷ���1��4-�ӳɺ�1��2-�ӳɣ���EΪ��ϩ���������Ƶ�C2Ϊ��CH3��2C=C��CH3��2��DΪ![]() ������������ӭ�ж����ˡ�
������������ӭ�ж����ˡ�

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�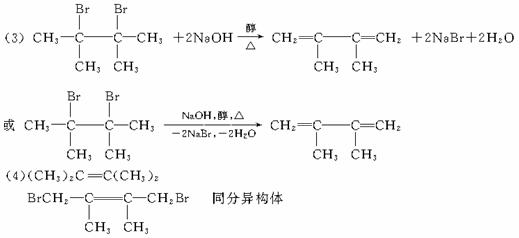





 +NaOH
+NaOH  CH3-CH=CH2��+NaCl+ H2O���÷�ӦʽҲ�ɱ�ʾΪ
CH3-CH=CH2��+NaCl+ H2O���÷�ӦʽҲ�ɱ�ʾΪ CH3-CH=CH2 ��ͼ�ǰ˸��л��������ת����ϵ
CH3-CH=CH2 ��ͼ�ǰ˸��л��������ת����ϵ 