��Ŀ����
����Ŀ����ʽ̼��ͭ[xCuCO3��yCu(OH)2]���ʿ�ȸ����ɫ.�ֳ�Ϊ��ȸʯ����һ������Ŀ��ﱦʯ������ͭ������е�������������̼��ˮ���������ʷ�Ӧ���������ʡ�CuSO4��Һ��Na2CO3��Һ��Ӧ���Եõ���ʽ̼��ͭ�����ǽ�������ɽ������̽����
[�����Ʊ�]
��ȡ12. 5 g����(CuSO4 5H2O)����87. 5mL����ˮ�У��μ�����ϡ����(������Ժ��Բ���)����ֽ����õ�CuSO4��Һ�������м���Na2CO3��Һ������������ɫ����Һ���ˣ���������ˮϴ�ӣ�������ˮ�Ҵ�ϴ�ӣ����͜غ�ɱ��á�
[ʵ��̽��]�������������װ�ã����Ƶõ�����ɫ�������ʵ�顣

��������ʵ��ش���������
(1)��������ͭ��Һ�Ĺ����еμ�ϡ�����������___________����������ͭ��Һ��������������Ϊ_________
(2)ʵ����ͨ��ʹ�ü����������ƺ��Ȼ�炙����Һ�ķ�����ȡN2���÷�Ӧ�Ļ�ѧ����Ϊ��__________��
��Dװ�ü���ǰ����Ҫ���ȴ���K��ͨ������N2��Ȼ��ر�K���ٵ�ȼD���ƾ��ơ�ͨ��N2������___________�� BΪ��ȫƿ��������ԭ��Ϊ_________��C��ʢװ���Լ�Ӧ��__________��
(4)����D��۲쵽��������________________��
(5)����������֪��Ksp[CaCO3]=2.8��10-9��Ksp[BaCO3]=5.1��10-9����������Ϊ��Ҫ��Ba(OH)2��Һ�������ʯ��ˮ�������ⶨ����ɫ����Ļ�ѧʽ����ԭ����______________
a.Ba(OH)2�ļ��Ա�Ca(OH)2ǿ
b.Ba(OH)2�ܽ�ȴ���Ca(OH)2���ܳ������CO2
c.��ͬ�����£�CaCO3���ܽ�����Դ���BaCO3
d.���յ���CO2���ɵ�BaCO3����������CaCO3���������С
(6)��D�з�Ӧ��ȫ����K���ٴεμ�NaNO2��Һ����N2����Ŀ����______________����װ��F��ʹ��Ba(OH)2��Һ��ʵ�����������װ��E����������0.27 g��F�в�������1.97 g���������ɫ����Ļ�ѧʽΪ_____________��[д��xCuCO3��yCu(OH)2����ʽ]
���𰸡�������Cu2+ˮ�⣬��ֹ��Һ����� 8.0%
��NaNO2+NH4Cl![]() NaCl+N2��+2H2O
NaCl+N2��+2H2O
���ų�װ���еĿ��� ��װ����ѹ������ʱ��Bƿ�м�İ�ȫ����Һ��������ʹѹ���ȶ� Ũ����
��Ӳ�ʲ�����������ɫ������ɫ��E�а�ɫ���������F����Һ�����
��bd
����ͣ����װ���е����屻������գ���Сʵ����� 2CuCO3��3Cu(OH)2
�������������⿼��ʵ����Ʒ��������ۣ���1��CuSO4����ǿ�������Σ���Һ�д���Cu2����H2O ![]() Cu(OH)2��H�����μ�ϡ���ᣬH��Ũ����������Cu2����ˮ�⣬��˵μ�ϡ���������������Cu2��ˮ�⣬��ֹ��Һ����ǣ����ʵ�����m(CuSO4)=12.5��160/250g=8g����Һ������Ϊ(12.5��87.5)g=100g������������Ϊ8/100��100%=8%����2��NaNO2��NH4Cl����������ԭ��Ӧ��NaNO2��NH4Cl��NaCl��N2����H2O��NaNO2��N���ϼ��ɣ�3�ۡ�0�ۣ�NH4Cl��N�Ļ��ϼ��ɣ�3�ۡ�0�ۣ����ݻ��ϼ۵�������������ƽ��Ȼ�����ԭ���غ���ƽ����������Ӧ����ʽΪNaNO2+NH4Cl
Cu(OH)2��H�����μ�ϡ���ᣬH��Ũ����������Cu2����ˮ�⣬��˵μ�ϡ���������������Cu2��ˮ�⣬��ֹ��Һ����ǣ����ʵ�����m(CuSO4)=12.5��160/250g=8g����Һ������Ϊ(12.5��87.5)g=100g������������Ϊ8/100��100%=8%����2��NaNO2��NH4Cl����������ԭ��Ӧ��NaNO2��NH4Cl��NaCl��N2����H2O��NaNO2��N���ϼ��ɣ�3�ۡ�0�ۣ�NH4Cl��N�Ļ��ϼ��ɣ�3�ۡ�0�ۣ����ݻ��ϼ۵�������������ƽ��Ȼ�����ԭ���غ���ƽ����������Ӧ����ʽΪNaNO2+NH4Cl![]() NaCl+N2��+2H2O����3������ǰ��ͨ��N2��Ŀ�����ų�װ���п�����װ��BΪ��ȫƿ��������ԭ���ǵ�װ����ѹ������ʱ��Bƿ�м�İ�ȫ����Һ��������ʹѹ���ȶ�������ʵ��Ŀ�ģ���Ҫ��֤����ɫ�������ȵIJ�����N2�е�ˮ�����Ժ���ʵ��������ţ������ȥ��װ��C�������dz�ȥN2�е�ˮ��������װ��Cʢ�ŵ��Լ���Ũ�����4��������CuO��H2O��CO2��Ӳ�ʲ�����������ɫ������ɫ��E�а�ɫ���������F����Һ����ǣ���5����Ba(OH)2��Һ����Ca(OH)2��ԭ���ǣ�Ba(OH)2���ܽ�ȴ���Ca(OH)2�ܽ�ȣ��ܳ������CO2��Ba�����ԭ����������Ca�����ԭ����������BaCO3��Ħ����������CaCO3��Ħ�����������ٳ���ʱ����������ѡ��bd��ȷ����6����Ӧ��������ͨ�뵪����������ͣ����װ���е����屻������գ���Сʵ����װ��E�������ӵ���H2O����������n(H2O)=0.27/18mol=0.015mol��F�г�����BaCO3����n(CO2)=1.97/197mol=0.01mol��HԪ��������Cu(OH)2����Cu(OH)2�����ʵ���Ϊ0.015mol��C������CuCO3����CuCO3�����ʵ���Ϊ0.01mol��x:y=0.01��0.015=2��3����2CuCO3��3Cu(OH)2��
NaCl+N2��+2H2O����3������ǰ��ͨ��N2��Ŀ�����ų�װ���п�����װ��BΪ��ȫƿ��������ԭ���ǵ�װ����ѹ������ʱ��Bƿ�м�İ�ȫ����Һ��������ʹѹ���ȶ�������ʵ��Ŀ�ģ���Ҫ��֤����ɫ�������ȵIJ�����N2�е�ˮ�����Ժ���ʵ��������ţ������ȥ��װ��C�������dz�ȥN2�е�ˮ��������װ��Cʢ�ŵ��Լ���Ũ�����4��������CuO��H2O��CO2��Ӳ�ʲ�����������ɫ������ɫ��E�а�ɫ���������F����Һ����ǣ���5����Ba(OH)2��Һ����Ca(OH)2��ԭ���ǣ�Ba(OH)2���ܽ�ȴ���Ca(OH)2�ܽ�ȣ��ܳ������CO2��Ba�����ԭ����������Ca�����ԭ����������BaCO3��Ħ����������CaCO3��Ħ�����������ٳ���ʱ����������ѡ��bd��ȷ����6����Ӧ��������ͨ�뵪����������ͣ����װ���е����屻������գ���Сʵ����װ��E�������ӵ���H2O����������n(H2O)=0.27/18mol=0.015mol��F�г�����BaCO3����n(CO2)=1.97/197mol=0.01mol��HԪ��������Cu(OH)2����Cu(OH)2�����ʵ���Ϊ0.015mol��C������CuCO3����CuCO3�����ʵ���Ϊ0.01mol��x:y=0.01��0.015=2��3����2CuCO3��3Cu(OH)2��

����Ŀ������ͼ��ʾװ�ü�����ϩʱ����Ҫ���ӵ���

��ϩ���Ʊ� | �Լ�X | �Լ�Y | |
A | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | KMnO4������Һ |
B | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | Br2��CCl4��Һ |
C | CH3CH2OH��ŨH2SO4������170�� | NaOH��Һ | KMnO4������Һ |
D | CH3CH2OH��ŨH2SO4������170�� | NaOH��Һ | Br2��ˮ��Һ |
A. A B. B C. C D. D
����Ŀ��ͨ������������������������ˮ�����ٷ�Һ�ŷŶԻ�������Ⱦ��ͬʱ����K2Cr2O7��ʵ���ҶԺ�����Һ(����Cr3+��Fe3+��K+��SO42����NO3��������Cr2O72��)�����������ù������£�

��֪����Cr(OH)3 + OH�� = CrO2�� + 2H2O��
��2CrO2�� + 3H2O2 + 2OH�� = 2CrO42�� + 4H2O��
��H2O2�����������¾��л�ԭ�ԣ��ܽ�+6��Cr��ԭΪ+3��Cr��
��1����ͼ����KOH��������250mL 6 mol��L��1 KOH��Һ�Ĺ���ʾ��ͼ��

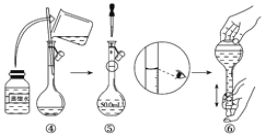
������۲�ͼʾ�жϣ����в���ȷ�IJ�����(�����)_____________________��
����������250 mL��Һ�����������(������)_________________��
�������ͼʾ�IJ���������Һ�������Ƶ���ҺŨ�Ƚ�________(�ƫ��ƫС��)��
(2)��Һ���ữǰ�����м��ȵ�Ŀ����____________________________����ԡ�����˺�Ӧ��������ˮϴ��K2Cr2O7����Ŀ����_______��
��3���±���������ʵ��ܽ�����ݣ�
���� | 0�� | 20�� | 40�� | 60�� | 80�� | 100�� |
KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 | K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
�����ܽ�����ݣ�����������������Ϊ��________________��________________��
��ȡ��Ʒ�ظ��������2.000g���250mL��Һ��ȡ��25.00mL����ƿ�У�����10mL 2mol��L��1H2SO4�������⻯��(���Ļ�ԭ����ΪCr3+�������ڰ���5min��Ȼ�����100mLˮ�� ����3mL����ָʾ������0.1200 mol��L��1Na2S2O3����Һ�ζ�(I2+2S2O32��=2I��+S4O62��)��
��д���ظ���������⻯�Ƶ����ӷ���ʽ_______________________��
�ڵζ��յ������Ϊ_________________________��
����ʵ���й���ȥNa2S2O3����Һ30.00mL�����ò�Ʒ�е��ظ���صĴ��� Ϊ_________(�������������������ʲ����뷴Ӧ)��
�����ζ�����ʹ��ǰδ��Na2S2O3����Һ��ϴ����õ��ظ���صĴ��Ƚ�_____________(�ƫ�ߡ�����ƫ�͡����䡱)��


