��Ŀ����
 Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ��ҵ�������У������Ź㷺�ġ���������ľ����ã�
Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ��ҵ�������У������Ź㷺�ġ���������ľ����ã���1��Nλ�����ڱ��е�
��2��Cu�������õĵ��硢���Ⱥ���չ�ԣ������Cu���е����Ե�ԭ��
��3����Cu�Ĵ������£��Ҵ��ɱ���������Ϊ��ȩ����ȩ������̼ԭ�ӵ��ӻ���ʽ��
��4��Cu+�ĵ����Ų�ʽΪ
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ���Ի���[Cu��H2O��2��Cl��2]���м��Եķ��ӵĽṹʽ
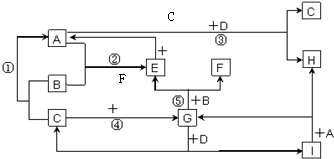
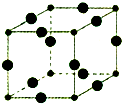
��6��Cu3N�ľ����ṹ��ͼ��N3-����λ��Ϊ
���㣺�����ļ���,ԭ�ӹ���ӻ���ʽ���ӻ������ж�
ר�⣺��ѧ���뾧��ṹ
��������1��Nλ�����ڱ��еڶ����ڵ�VA�壬��N3-������ͬ����������Ϊ�ȵ����壬��NO2-���ȵ�����ṹ���ƣ����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
��2���������ɵ��ӵĽ��������ܵ��磻
��3����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ����ݴ��ж�̼ԭ�ӵ��ӻ���ʽ��̼ԭ���ӻ���ʽ��ͬ��������Dz�ͬ��
��4��Cu+�ĺ�����28�����ӣ����ݹ���ԭ����д���̬���Ӻ�������Ų�ʽ��ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ���
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ��[Cu��H2O��2��Cl��2]���м��Եķ��ӣ�˵���÷��ӵĽṹ���Գƣ�
��6��Cu3N�ľ����ṹ��ͼ���������=12��
=3�������=
��8=1�����Դ����ʾCuԭ�ӡ�С���ʾNԭ�ӣ�N3-����λ��=3��2=6��Cu3N���ܶ�=
��
��2���������ɵ��ӵĽ��������ܵ��磻
��3����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ����ݴ��ж�̼ԭ�ӵ��ӻ���ʽ��̼ԭ���ӻ���ʽ��ͬ��������Dz�ͬ��
��4��Cu+�ĺ�����28�����ӣ����ݹ���ԭ����д���̬���Ӻ�������Ų�ʽ��ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ���
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ��[Cu��H2O��2��Cl��2]���м��Եķ��ӣ�˵���÷��ӵĽṹ���Գƣ�
��6��Cu3N�ľ����ṹ��ͼ���������=12��
| 1 |
| 4 |
| 1 |
| 8 |
| m |
| V |
���
�⣺��1��Nλ�����ڱ��еڶ����ڵ�VA�壬��N3-������ͬ����������Ϊ�ȵ����壬��NO2-���õ�����ṹ���ƣ��������������Nԭ�Ӽ۲���ӶԸ���=2+
����5+1-2��2��=3�Һ���һ���µ��Ӷԣ�����ΪV�νṹ���ʴ�Ϊ������VA��V��
��2��ͭ���ڽ������壬�����к��п��������ƶ��ĵ��ӣ�ͨ������ƶ��������ܵ��磬
�ʴ�Ϊ��CuΪ�������壬�����д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
��3����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ������Լ��е�̼ԭ�Ӳ���sp3�ӻ���ȩ���е�̼ԭ�Ӳ���sp2�ӻ���ȩ����̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ���������ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ��ǣ�
�ʴ�Ϊ��sp3��sp2�����ڣ�
��4��Cu+�ĺ�����28�����ӣ����ݹ���ԭ��֪���̬���Ӻ�������Ų�ʽ1s22s22p63s23p63d10��ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ���Cu+��3d�����ȫ�����ȶ����ʴ�Ϊ��1s22s22p63s23p63d10��Cu+��3d����ϵ���ȫ����ṹ�ȶ���
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ��[Cu��H2O��2��Cl��2]���м��Եķ��ӣ�˵���÷��ӵĽṹ���Գƣ�����ṹʽΪ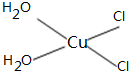 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��6��Cu3N�ľ����ṹ��ͼ���������=12��
=3�������=
��8=1�����Դ����ʾCuԭ�ӡ�С���ʾNԭ�ӣ�N3-����λ��=3��2=6�����������=[��2a+2b����10-10cm]3��Cu3N���ܶ�=
=
g/cm3=
g/cm3���ʴ�Ϊ��6��
��
| 1 |
| 2 |
��2��ͭ���ڽ������壬�����к��п��������ƶ��ĵ��ӣ�ͨ������ƶ��������ܵ��磬
�ʴ�Ϊ��CuΪ�������壬�����д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
��3����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ������Լ��е�̼ԭ�Ӳ���sp3�ӻ���ȩ���е�̼ԭ�Ӳ���sp2�ӻ���ȩ����̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ���������ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ��ǣ�
�ʴ�Ϊ��sp3��sp2�����ڣ�
��4��Cu+�ĺ�����28�����ӣ����ݹ���ԭ��֪���̬���Ӻ�������Ų�ʽ1s22s22p63s23p63d10��ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ���Cu+��3d�����ȫ�����ȶ����ʴ�Ϊ��1s22s22p63s23p63d10��Cu+��3d����ϵ���ȫ����ṹ�ȶ���
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ��[Cu��H2O��2��Cl��2]���м��Եķ��ӣ�˵���÷��ӵĽṹ���Գƣ�����ṹʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����6��Cu3N�ľ����ṹ��ͼ���������=12��
| 1 |
| 4 |
| 1 |
| 8 |
| m |
| V |
| ||
| [(2a+2b)��10-10]3 |
| 103��1030 |
| 4(a+b)3NA |
| 103��1030 |
| 4(a+b)3NA |
���������⿼�������ʽṹ�����ʣ��漰�����ļ��㡢ԭ���ӻ�����������Ų���֪ʶ�㣬�����ܶȹ�ʽ���۲���ӶԻ������ۡ�����ԭ����֪ʶ�������������Щ֪ʶ�㶼�ǿ����ȵ㣬�ѵ��Ǿ����ļ��㣬��ȷ������ĸ�ĺ��壬ע�⣨1���в���֪ʶǨ�Ƶķ������н����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
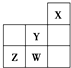 ������Ԫ��W��X��Y��Z��Ԫ�����ڱ��е�λ����ͼ��ʾ������˵���в���ȷ���ǣ�������
������Ԫ��W��X��Y��Z��Ԫ�����ڱ��е�λ����ͼ��ʾ������˵���в���ȷ���ǣ�������| A��W������������Ӧ��ˮ������ǿ�� |
| B��Y��ԭ�Ӱ뾶��ͬ��������Ԫ������С |
| C��W�ķǽ����Ա�Z��ǿ |
| D��Z����̬�⻯����ȶ�����ͬ����Ԫ������ǿ |
��ϩ�ܱ����Ը��������Һ����Ϊ������̼���������л�����ϩ������ȥ��ϩ�õ����﴿�������飬���ͨ��ʢ�����������Լ���ϴ��ƿ��������
| A�������ʯ��ˮ��Ũ���� |
| B�����Ը��������Һ��Ũ���� |
| C����ˮ��Ũ���� |
| D��Ũ���ᡢ���Ը��������Һ |
��һ������п��100mL 18.5mol/L H2SO4��ַ�Ӧ��п��ȫ�ܽ⣬ͬʱ��������A 22.4L����״����������Ӧ�����Һϡ����1L�������Һ��pH=1�������������д�����ǣ�������
| A������AΪSO2��H2�Ļ���� |
| B����Ӧ�й�����Zn 65g |
| C������A��SO2��H2�������Ϊ1��4 |
| D����Ӧ�й�ת�Ƶ���2mol |
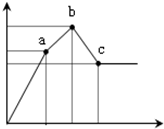 ��100mL0.1mol/L�������[NH4Al��SO4��2]��Һ����ε���0.1mol/L Ba��OH��2��Һ������Ba��OH��2��Һ���V�������꣩�ı仯�����������ʵ���n�ı仯��ͼ��ʾ��
��100mL0.1mol/L�������[NH4Al��SO4��2]��Һ����ε���0.1mol/L Ba��OH��2��Һ������Ba��OH��2��Һ���V�������꣩�ı仯�����������ʵ���n�ı仯��ͼ��ʾ��