��Ŀ����
д�����и��л���Ľṹ��ʽ����1����0.2mol��A����������ȫȼ��ʱ����CO2��H2O��1.2mol�������������2��2-�������飬��A�ĽṹʽΪ__________________��
��2��ijȲ����H2��ּӳ�����2��5-�������飬��Ȳ���Ľṹ��ʽΪ______________��
��3��ij��1mol��2mol HCl��ȫ�ӳɣ����ɵ��ȴ�������������4mol������Ӧ��������Ľṹ��ʽΪ________________��
��4��ij����A�������ܶ�����ͬ״���������ܶȵ�64�������ⶨ��֪A�����й���6��������A��������ϩ���������ӳɵIJ��A�Ľṹ��ʽΪ_____________��
�𰸣�
������
������

��1������̼ԭ�����ֻ�����ĸ�ԭ�������ӣ��磺
��3�����ɵ��ȴ��������ֻ��4��Hԭ�ӡ� ��4������������������Ҳ�������CH3��CH2��CH2����2������
|

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ




 ���������ŵ�������
���������ŵ�������
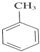 +3HNO3
+3HNO3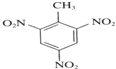 +3H2O��
+3H2O��