��Ŀ����
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش���������
��1����Ũ������HCl�����ʵ���Ũ��Ϊ______mol/L��
��2������250mL 0.1mol/L��������Һ
A������Ͳ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ���ò���������������ʹ���Ͼ��ȣ�
B��������ȴ�������ز�����ע������ƿ�У�
C��������ˮϴ��______2-3�Σ�ϴ��Һ��ע������ƿ����
D������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��������______��ˮ��ʹ��Һ����ǡ����̶����У�
E��������ƿ�ǽ�����ҡ�ȣ�
��3����ʵ���г������������������ҺŨ����ʲôӰ�죿����ƫ�ߡ�ƫ�͡���Ӱ�죩
�ٽ��ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ______��
��������ƿ��ת����Һʱ������Һ�ε�������ƿ���棬��Ũ��______��
�۶���ʱ��������ƿ�̶���______��
��4��ʵ������Ѹ���Ʊ������������������·�Ӧ��
2KMnO4+16HCl�T2KCl+2MnCl2+5Cl2��+8H2O
�˷�Ӧ����Ҫ���ȣ������¾Ϳ���Ѹ�ٽ��У�
���á�˫���ŷ�����������ת�Ƶķ������Ŀ��
2KMnO4+16HCl�T2KCl+2MnCl2+5Cl2��+8H2O
���ø�Ũ�����Ƶ��˱����560mlCl2����������HClΪ______mol����ҪKMnO4������______g��
��1����Ũ������HCl�����ʵ���Ũ��Ϊ______mol/L��
��2������250mL 0.1mol/L��������Һ
| Ӧ��ȡŨ�������/mL | Ӧѡ������ƿ�Ĺ��/mL |
| ______ | ______ |
B��������ȴ�������ز�����ע������ƿ�У�
C��������ˮϴ��______2-3�Σ�ϴ��Һ��ע������ƿ����
D������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��������______��ˮ��ʹ��Һ����ǡ����̶����У�
E��������ƿ�ǽ�����ҡ�ȣ�
��3����ʵ���г������������������ҺŨ����ʲôӰ�죿����ƫ�ߡ�ƫ�͡���Ӱ�죩
�ٽ��ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ______��
��������ƿ��ת����Һʱ������Һ�ε�������ƿ���棬��Ũ��______��
�۶���ʱ��������ƿ�̶���______��
��4��ʵ������Ѹ���Ʊ������������������·�Ӧ��
2KMnO4+16HCl�T2KCl+2MnCl2+5Cl2��+8H2O
�˷�Ӧ����Ҫ���ȣ������¾Ϳ���Ѹ�ٽ��У�
���á�˫���ŷ�����������ת�Ƶķ������Ŀ��
2KMnO4+16HCl�T2KCl+2MnCl2+5Cl2��+8H2O
���ø�Ũ�����Ƶ��˱����560mlCl2����������HClΪ______mol����ҪKMnO4������______g��

��1����Һ���������������ʵ���Ũ��֮��Ĺ�ϵ��ʽc=
=
=12mol/L���ʴ�Ϊ��12��
��2��������ϡ��ǰ�����ʵ��������ֲ��䣬����ҪŨ��������ΪVmL����1.2��V��36.5%=0.25��0.1�����V=2.1mL����Һ�����������ƿ�Ĺ���������ѡ��250mL������ƿ��������ܽ�ʹ���ٰף�����ʱ���ý�ͷ�ιܣ��ʴ�Ϊ��2.1��250���ձ�����ͷ�ιܣ�
��3���ٽ��ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ����Ҫ���ж��ݣ����Զ�ʵ������Ӱ�죻
��������ƿ��ת����Һʱ������Һ�ε�������ƿ���棬��ô��������ʧ�����Ե��������������٣�����Ũ��ƫС��
�۶���ʱ��������ƿ�̶��ߣ�������Һ�������С��������ҺŨ��ƫ��
�ʴ�Ϊ������Ӱ�죻 ��ƫ�ͣ���ƫ�ߣ�
��4����������ԭ��Ӧ�У����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ�����=10������ת��������£�

��
�ʴ�Ϊ��

��
���ڷ�Ӧ�У�ÿ����5mol���������ͻ���16mol������μӷ�Ӧ��������ֻ�ԭ�Ժ����ԣ�������10mol��������ֻ�ԭ�ԣ�������������������0.025molʱ�������ĵ��������ʵ���Ϊ
0.08mol��������������Ϊ0.05 mol�����ĸ�����ص�������0.01mol��316g/mol=3.16g���ʴ�Ϊ��0.05��3.16��
| 1000��w% |
| M |
| 1000��1.2g/cm3��36.5% |
| 36.5g/mol |
��2��������ϡ��ǰ�����ʵ��������ֲ��䣬����ҪŨ��������ΪVmL����1.2��V��36.5%=0.25��0.1�����V=2.1mL����Һ�����������ƿ�Ĺ���������ѡ��250mL������ƿ��������ܽ�ʹ���ٰף�����ʱ���ý�ͷ�ιܣ��ʴ�Ϊ��2.1��250���ձ�����ͷ�ιܣ�
��3���ٽ��ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ����Ҫ���ж��ݣ����Զ�ʵ������Ӱ�죻
��������ƿ��ת����Һʱ������Һ�ε�������ƿ���棬��ô��������ʧ�����Ե��������������٣�����Ũ��ƫС��
�۶���ʱ��������ƿ�̶��ߣ�������Һ�������С��������ҺŨ��ƫ��
�ʴ�Ϊ������Ӱ�죻 ��ƫ�ͣ���ƫ�ߣ�
��4����������ԭ��Ӧ�У����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ�����=10������ת��������£�

��
�ʴ�Ϊ��

��
���ڷ�Ӧ�У�ÿ����5mol���������ͻ���16mol������μӷ�Ӧ��������ֻ�ԭ�Ժ����ԣ�������10mol��������ֻ�ԭ�ԣ�������������������0.025molʱ�������ĵ��������ʵ���Ϊ
0.08mol��������������Ϊ0.05 mol�����ĸ�����ص�������0.01mol��316g/mol=3.16g���ʴ�Ϊ��0.05��3.16��

��ϰ��ϵ�д�
�����Ŀ
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺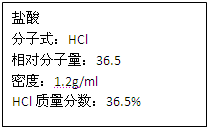 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺ ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺