��Ŀ����
����Ŀ����������(CaO2)��һ�ְ�ɫ���壬�ܳ��⣬������ˮ������ˮ������Ӧ���������Ҵ��������ᷴӦ��������ɱ�������������ȡ��������⣬�ش�������⡣
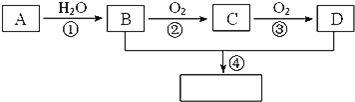
I��CaO2������Ʊ���CaO2����ͨ��������CaCl2�ڼ�����������H2O2��Ӧ�Ƶá�ij��ѧ��ȤС����ʵ�����Ʊ�CaO2��ʵ�鷽����װ��ʾ��ͼ���£�


(1)������ƿ�з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ___________��
(2)��ˮԡ��Ŀ����__����������ϴ��CaO2��8H2O��ʵ�����������__
����CaO2�����IJⶨ���ⶨCaO2��Ʒ���ȵķ����ǣ���ȡ0.200g��Ʒ����ƿ�У�����50mLˮ��15mL2mol��L-lHCl����ʹ��Ʒ�ܽ����ɹ������⣬�ټ��뼸��MnCl2ϡ��Һ��������0.0200mol��L-lKMnO4����Һ�ζ����յ㣬����25.00mL��Һ��
(3)����������ʹ��ϡ�������ʹ��ϡ�����ܽ���Ʒ��ԭ����______���ζ�ǰ����MnCl2ϡ��Һ�����ÿ�����______��
(4)�ζ������е����ӷ���ʽΪ____����Ʒ��CaO2����������Ϊ____��
(5)ʵ��I�Ƶõľ�����Ʒ��CaO2����ƫ�͵Ŀ���ԭ���ǣ�_______��
���𰸡�CaCl2+H2O2+2NH3��H2O+6H2O=CaO2��8H2O��+2NH4Cl �÷�Ӧ���ȣ���ֹ�¶����ߵ���H2O2�ֽ�Ͱ�ˮ�а����ӷ� ���������ע���Ҵ�����û���������Ҵ��������ظ�����2��3�� ���ɵ������Ϊ�����������Ʒ���棬��ֹ��Ӧ��һ������ ������ ![]() 45.0% ����CaCl2��Һ��Ũ��ˮ��Ӧ����Ca(OH)2 ������CaO2��ˮ��Ӧ����Ca(OH)2��濾CaO2��8H2Oʧˮ������ȫ
45.0% ����CaCl2��Һ��Ũ��ˮ��Ӧ����Ca(OH)2 ������CaO2��ˮ��Ӧ����Ca(OH)2��濾CaO2��8H2Oʧˮ������ȫ
��������
(1)����������ԭ��Ӧ��ԭ���غ������д��Ӧ����ʽ��
(2) ����H2O2�����ֽ⣬��ˮ�����ӷ���������⣻
(3)������������(CaO2)��һ�ְ�ɫ���壬�����ᷴӦ�����ʽ��з������
(4)����������ԭ��Ӧ��ʧ�����غ㡢����غ㡢ԭ���غ���н��
(5)��Ϊ����CaCl2��Һ��Ũ��ˮ��Ӧ����Ca(OH)2������CaO2��ˮ��Ӧ����Ca(OH)2��濾CaO2��8H2Oʧˮ������ȫ������Ʒ��CaO2����ƫ�͡�
(1)�����֪��������ƿ��CaCl2�ڼ�����������H2O2������Ӧ����CaO2��8H2O����ԭ���غ��֪���÷�Ӧ��ѧ����ʽΪ��CaCl2+H2O2+2NH3��H2O+6H2O=CaO2��8H2O��+2NH4Cl���ʴ𰸣�CaCl2+H2O2+2NH3��H2O+6H2O=CaO2��8H2O��+2NH4Cl��
(2) H2O2�����ֽ⣬��ˮ�����ӷ����÷�ӦΪ���ȷ�Ӧ��H2O2�Ͱ�ˮ�����ȶ��Զ��ϲ�¶ȹ��ᵼ����ֽ⣬Ӱ������ͻ�ѧ��Ӧ���ʣ���������ƿ���ˮ������Ӧ���������Ҵ�����˿�ѡ���Ҵ�����ϴ�ӣ�ʵ����ϴ�ӳ����IJ���Ϊ�����������ע���Ҵ�����û���������Ҵ��������ظ�����2~3�Σ��ʴ�Ϊ���÷�Ӧ���ȣ���ֹ�¶����ߵ���H2O2�ֽ�Ͱ�ˮ�а����ӷ������������ע���Ҵ�����û����,���Ҵ�������,�ظ�����2~3�Σ�
(3)��ѡ��ϡ������CaO2��ϡ���ᷴӦ��������CaSO4�Ḳ������Ʒ���棬ʹ��Ӧ���Գ������У�MnCl2�Ը÷�Ӧ���д����ã��ɼӿ컯ѧ��Ӧ���ʣ��ʴ�Ϊ����������CaSO4�Ḳ������Ʒ���棬ʹ��Ӧ���Գ������У������ã�
(4)�ζ����������Ը��������˫��ˮ��Ӧ,MnԪ�ػ��ϼ۴�+7�۽�����+2�ۣ�H2O2��OԪ�ش�-1��������0�ۣ�����������ԭ��Ӧ��ʧ�����غ㡢����غ㡢ԭ���غ���ƽ�����ӷ���ʽΪ��![]() ���ζ����������ĸ�����ص����ʵ���n=25
���ζ����������ĸ�����ص����ʵ���n=25![]() 10-3L
10-3L![]() 0.02mol/L=5
0.02mol/L=5![]() 10-4 mol�������غ��ϵ��֪��n(H2O2)=n(CaO2)=2.5n(KMnO4)=1.25
10-4 mol�������غ��ϵ��֪��n(H2O2)=n(CaO2)=2.5n(KMnO4)=1.25![]() 10-3mol����Ʒ��CaO2����������w(CaO2)=
10-3mol����Ʒ��CaO2����������w(CaO2)=![]()
![]() 100%=45%���ʴ�Ϊ��
100%=45%���ʴ�Ϊ�� ![]() ��45%��
��45%��
(5)����CaCl2��Һ��Ũ��ˮ��Ӧ���·�Ӧ��δ��ȫת����ͬʱ���ᵼ�����չ����к��в���Ca(OH)2���ʣ���ʹCaO2����ƫ�ͣ�����CaO2��ˮ�ܷ�Ӧ��������Ca(OH)2�ᵼ�����ɵ�CaO2����ƫ�ͣ��濾CaO2��8H2Oʧˮ������ȫ���¹�������ƫ�����յ��¼���CaO2����ƫ�ͣ��ʴ�Ϊ������CaCl2��Һ��Ũ��ˮ��Ӧ����Ca(OH)2������CaO2��ˮ��Ӧ����Ca(OH)2��濾CaO2��8H2Oʧˮ������ȫ��

����Ŀ��Ni(CO)4�������л��ϳɣ�Ҳ������������һ�������£������ܱ������з�����ӦNi(s)��4CO(g)![]() Ni(CO)4(g)���÷�Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���±���ʾ��
Ni(CO)4(g)���÷�Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���±���ʾ��
�¶�/�� | 25 | 80 | 230 |
ƽ�ⳣ�� | 5��104 | 2 | 1.9��10-5 |
����˵����ȷ����
A.25��ﵽƽ��ʱ���������м�������CO(g)��CO��ת���ʼ�С
B.�¶�Խ�ͣ�Խ������Ni(CO)4������
C.80��ʱ�����ijʱ��Ni(CO)4��CO��Ũ�Ⱦ�Ϊ0.5 mol��L-1�����ʱv����v��
D.ƽ������ܱ������м����������ۣ�ƽ�������ƶ����ﵽ��ƽ��ʱCO��Ũ�ȱ�ԭƽ��С
����Ŀ������ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�

CH3CH2CH2CH2OH![]() CH3CH2CH2CHO ����Ӧ��Ͳ������������б����£�
CH3CH2CH2CHO ����Ӧ��Ͳ������������б����£�
�е�/�� | �ܶ�/(g��cm��3) | ˮ���ܽ��� | |
������ | 117.2 | 0.810 9 | �� |
����ȩ | 75.7 | 0.801 7 | �� |
ʵ�鲽�����£���6.0 g Na2Cr2O7����100 mL�ձ��У���30 mLˮ�ܽ⣬��5 mLŨ�����γɻ����Һ����������ҺС��ת����B�С���A�м���4.0 g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95 �棬��E���ռ�90 �����µ���֡�������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77 ����֣�����2.0 g��
�ش��������⣺
(1)ʵ���У�Na2Cr2O7��Һ��Ũ�������ӵ�˳��Ϊ___________________________��
(2)�����ʯ��������________________________________________________��
�����Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������______________________________��
(3)����װ��ͼ�У�D������������________��E������������________��
(4)��Һ©��ʹ��ǰ������еIJ�����________��
(5)������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ������ȩ��_______________��(��ϡ����¡�)��
(6)��Ӧ�¶�Ӧ������90��95 �棬��ԭ����__________________________________��__________________________________________________��
(7)��ʵ���У�����ȩ�IJ���Ϊ________%�����������λС������