��Ŀ����
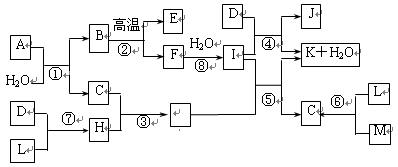
��14�֣���ͼ��ʾ����������1~20��Ԫ���в���Ԫ����ɵĵ��ʻ��仯���ͼ�в��ַ�Ӧ����δ�г�����֪C��H����ɫ�д̼�����ζ�����壬D��һ�ֻ���ɫ�����嵥�ʣ�����J������Ӿ����������Ӧ�ں͢��ǻ�����������Ҫ��Ӧ����Ӧ����ʵ�����Ʊ�����C����Ҫ������

��ش��������⣺
��1������E�ĵ���ʽ��__________��I��������ѧ������Ϊ��____________________������D����Ԫ�������ڱ��е�λ����____________________��
��2��G��ˮ��Һ��__________�ԣ������ӷ���ʽ��ʾ��ԭ��___________________________��[��Դ:ZXXK]
��3��д��E�����I��Һ��Ӧԭ���ӷ���ʽ______________________________��
��4��д��ʵ�����Ʊ�����C��Ӧ�Ļ�ѧ����ʽ______________________________����������C��ѡ��__________��������������ƣ���
��5��д����Ӧ�ܵĻ�ѧ����ʽ__________________________________________________��
��6����Ӧ�۵�����Ϊ______________________________��
��7����֪��7.4g I��ϡ��Һ��200mL 1 mol/L��H��Һ��Ӧ�ų�11.56kJ��������
д���÷�Ӧ���Ȼ�ѧ����ʽ________________________________________��
��8������A������Ԫ����ɣ�1 molA��ˮ��Ӧ������1 mol B��2 mol C��A��ѧʽΪ______________________________��
��1�� ���Ӽ������Լ� ��������VIIA��
���Ӽ������Լ� ��������VIIA��
��2���� NH4++H2O NH3��H2O+H+
NH3��H2O+H+
 ��3��CO2+Ca2++2OH-=CaCO3��+H2O
��3��CO2+Ca2++2OH-=CaCO3��+H2O
��4��2NH4Cl+Ca(OH)2===CaCl2+2NH3��+2H2O ��ʯ��
��5��2Cl2+2Ca(OH)2===CaCl2+Ca(ClO)2+2H2O
��6����������
��7��Ca(OH)2(aq)+2HCl(aq)===CaCl2(aq)+2H2O(l) ��H=-115.6kg/mol
��8��CaCN2
����������



 ���ݻ���ȵ��ܱ�������
���ݻ���ȵ��ܱ�������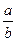 ________
________ 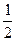 (ѡ�>������<����=������
(ѡ�>������<����=������