��Ŀ����
����������Ԫ��A��B��C��D��Eԭ��������������AΪ���ڱ��а뾶��С��Ԫ�أ�Bԭ�ӵ�����������������Ӳ�����2����D��Eͬ���壬E��ԭ��������D��2����
��1��A��CԪ�ؿ����γɶ��ֻ�������м��ǻ���ƽ����г��õ�Һ̬ȼ�ϣ�����A��CԪ�ص�������Ϊ7��1����֪0.4molҺ̬��������ȫ��Ӧ���ɵ�����Һ̬ˮ�ų�248.8kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ____________ ��
��2����ֱ����Һ̬��Ϊȼ�ϵĵ���У��������ҺΪNaOH��Һ�������ķ�ӦʽΪ___________������״̬�£���ȼ�ϵ������1molҺ̬�����ܲ�����������Ϊ528.7kJ�����ȼ�ϵ�ص�����Ч��Ϊ________��ȼ�ϵ�ص�����Ч����ָ�������������������ȼ�ϵ�ط�Ӧ�����ͷŵ�ȫ������֮�ȣ���
��3��A��B��DԪ�ؿ����γɻ������ң��仯ѧʽΪA2B2D4��0.2mol/L������Һ��0.2mol/L��NaOH��Һ�������ϣ�������Һ�����ԣ������Һ�и�����Ũ���ɴ�С���е�˳��Ϊ_______________ ��
��4����8mL 0.1mol/L�����Ը��������Һ��2mL 1.0mol/L������Һ���Թ��л�ϣ����Թ�����25��ˮ�У�����ͼ��ʾ����KMnO4Ũ����ʱ��仯��ϵ����ͼ��ʾ��
��1��A��CԪ�ؿ����γɶ��ֻ�������м��ǻ���ƽ����г��õ�Һ̬ȼ�ϣ�����A��CԪ�ص�������Ϊ7��1����֪0.4molҺ̬��������ȫ��Ӧ���ɵ�����Һ̬ˮ�ų�248.8kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ____________ ��
��2����ֱ����Һ̬��Ϊȼ�ϵĵ���У��������ҺΪNaOH��Һ�������ķ�ӦʽΪ___________������״̬�£���ȼ�ϵ������1molҺ̬�����ܲ�����������Ϊ528.7kJ�����ȼ�ϵ�ص�����Ч��Ϊ________��ȼ�ϵ�ص�����Ч����ָ�������������������ȼ�ϵ�ط�Ӧ�����ͷŵ�ȫ������֮�ȣ���
��3��A��B��DԪ�ؿ����γɻ������ң��仯ѧʽΪA2B2D4��0.2mol/L������Һ��0.2mol/L��NaOH��Һ�������ϣ�������Һ�����ԣ������Һ�и�����Ũ���ɴ�С���е�˳��Ϊ_______________ ��
��4����8mL 0.1mol/L�����Ը��������Һ��2mL 1.0mol/L������Һ���Թ��л�ϣ����Թ�����25��ˮ�У�����ͼ��ʾ����KMnO4Ũ����ʱ��仯��ϵ����ͼ��ʾ��

��д��������Ӧ�����ӷ���ʽ_______________ ��
�ڼ���ǰ40�����ұ�ʾ��ƽ����Ӧ���ʣ�v���ң�______________��
��40s~65s�ķ�Ӧ���ʱ�ǰ40s�죬����ԭ��_______________ ��
�ڼ���ǰ40�����ұ�ʾ��ƽ����Ӧ���ʣ�v���ң�______________��
��40s~65s�ķ�Ӧ���ʱ�ǰ40s�죬����ԭ��_______________ ��
��1��N2H4(l)+ O2(g) == N2(g) + 2H2O(l) ��H=-622 kJ/mol
��2��N2H4 + 4OH-+ 4e- == N2 + 4H2O ��85%
��3��Na+��HC2O4-��H+��C2O42-��OH-
��4����5H2C2O4 + 6H+ + 2MnO4- == 10CO2��+ 2Mn2+ + 8H2O
��1��10-3 mol/(L��s)
��Mn2+�Ǵ˷�Ӧ�Ĵ��������ŷ�Ӧ���У���ʼ����Mn2+����Ũ��Խ��Խ�����Է�Ӧ����Ѹ�ټӿ졣
��2��N2H4 + 4OH-+ 4e- == N2 + 4H2O ��85%
��3��Na+��HC2O4-��H+��C2O42-��OH-
��4����5H2C2O4 + 6H+ + 2MnO4- == 10CO2��+ 2Mn2+ + 8H2O
��1��10-3 mol/(L��s)
��Mn2+�Ǵ˷�Ӧ�Ĵ��������ŷ�Ӧ���У���ʼ����Mn2+����Ũ��Խ��Խ�����Է�Ӧ����Ѹ�ټӿ졣

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
����������Ԫ��A��B��C��D��ԭ��������������A��Cԭ���������8��A��B��C����Ԫ��ԭ�ӵ�����������֮��Ϊ15��Bԭ����������������Aԭ��������������һ�룮����������ȷ���ǣ�������
| A��ԭ�Ӱ뾶��A��D��C��B | B��B��C��D�ֱ���A�γɵĻ�����һ��������ͬ�Ļ�ѧ�� | C������������Ӧˮ��������ԣ�D��C | D�������£�����B�ܴ�������Ũ������ |
����������Ԫ��A��B��C��ԭ���������ε�����A��Cͬ���壬Bԭ�ӵ���������������Aԭ�ӵĴ���������������ԭ�ӵ�����������֮��Ϊ10��������������ȷ���ǣ�������
| A��ԭ�ڰ뾶A��B��C | B��A����̬�⻯���ȶ��Դ���C����̬�⻯���ȶ��� | C��A��C��Ԫ����������������ˮ���ϵõ���Ӧ���� | D������ʱ��A���ʿ��Դ�C�����������û��õ�C���� |
����������Ԫ��A��B��C��D��E��ԭ������������������A��Cͬ���壬A������Ԫ�ز���ͬһ���ڣ�B��Dͬ���壬������D�ĵ���Ϊ����ɫ���壮�����ƶ�����ȷ���ǣ�������
| A��ԭ�Ӱ뾶��С�����˳��r��C����r��D����r��E�� | B��Ԫ��D��E�ֱ���A�γɵĻ���������ȶ��ԣ�E��D | C��Ԫ��D������������Ӧˮ��������Ա�E��ǿ | D��Ԫ��B�ֱ���A��C�γɵĻ������л�ѧ����������ȫ��ͬ |
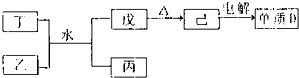
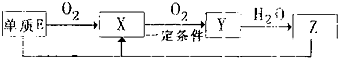
 ����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ����
����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ���� NH3?H2O+H+
NH3?H2O+H+ 2NO2
2NO2