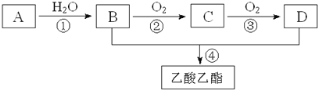
��Ŀ����
����Ŀ����X��Y��Z��W���ֺ�14�����ӵ����ӣ���ṹ�ص����£�
���Ӵ��� | X | Y | Z | W |
ԭ�Ӻ��� | ���� | ��ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� |
���ӵĵ���� | 0 | 0 | ��������� | 0 |
![]() ԭ�Ӻ����Xԭ�Ӷ�3�����ӣ�A��ԭ�ӽṹʾ��ͼ�� ______ ��
ԭ�Ӻ����Xԭ�Ӷ�3�����ӣ�A��ԭ�ӽṹʾ��ͼ�� ______ ��![]() �������ᄃ���к��й��ۼ���ĿΪ ______
�������ᄃ���к��й��ۼ���ĿΪ ______
![]() ���������ɵĻ�����ĵ���ʽΪ ______
���������ɵĻ�����ĵ���ʽΪ ______
![]() ��ȫȼ�շų���������
��ȫȼ�շų���������![]() ��д��Yȼ�յ��Ȼ�ѧ����ʽ ______
��д��Yȼ�յ��Ȼ�ѧ����ʽ ______
![]() ���W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ
���W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ![]() ��Ӧ������������������
��Ӧ������������������![]()
![]()
![]() д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ ______
д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ ______
![]() ���W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ���� ______ �����⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ ______ ��
���W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ���� ______ �����⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ ______ ��
���𰸡�![]()
![]()
![]()
![]() 3Fe+4H2O
3Fe+4H2O![]() Fe3O4+4H2��C+H2O
Fe3O4+4H2��C+H2O![]() CO+H2
CO+H2 ![]() ��
��![]() ��������
�������� ![]()
��������
X��Y��Z��W���ֺ�14�����ӵ����ӣ�XΪ���ˣ������Ϊ0����X��������Ϊ14��XΪSiԭ�ӣ�YΪ��ͬԪ�ع��ɵ��������ӣ����ӵ����Ϊ0����YΪCO��ZΪͬԪ�ع��ɵ��������ӣ����ӵ����Ϊ![]() �����γ�ZԪ�ص�ԭ��������Ϊ
�����γ�ZԪ�ص�ԭ��������Ϊ![]() ����ZΪ
����ZΪ![]() ��WΪͬԪ�ع��ɵ��������ӣ����ӵ����Ϊ0�����γ�WԪ�ص�ԭ��������Ϊ
��WΪͬԪ�ع��ɵ��������ӣ����ӵ����Ϊ0�����γ�WԪ�ص�ԭ��������Ϊ![]() ��ΪNԪ�أ�WΪ
��ΪNԪ�أ�WΪ![]() �������Ϸ������
�������Ϸ������
![]() ԭ�Ӻ����Siԭ�Ӷ�3�����ӣ�AΪClԭ�ӣ�ԭ�Ӻ��������Ϊ17����3�����Ӳ㣬���������Ϊ2��8��7��ԭ�ӽṹʾ��ͼΪ
ԭ�Ӻ����Siԭ�Ӷ�3�����ӣ�AΪClԭ�ӣ�ԭ�Ӻ��������Ϊ17����3�����Ӳ㣬���������Ϊ2��8��7��ԭ�ӽṹʾ��ͼΪ![]() ��
��![]() ������ÿ��Siԭ����4��Oԭ��֮���γ�4��
������ÿ��Siԭ����4��Oԭ��֮���γ�4��![]() ����
����![]() �������
�����к���![]() �����ۼ����ʴ�Ϊ��
�����ۼ����ʴ�Ϊ��![]() ��
��![]() ��
��
![]() ̼����Ϊ���ӻ�����ɸ�������
̼����Ϊ���ӻ�����ɸ�������![]() ���ӹ��ɣ�
���ӹ��ɣ�![]() ������Cԭ��֮���γ�3�Թ��õ��Ӷԣ�̼���Ƶ���ʽΪ��
������Cԭ��֮���γ�3�Թ��õ��Ӷԣ�̼���Ƶ���ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() �����������ʵ���Ϊ
�����������ʵ���Ϊ![]() ����2molCOȼ�շų�������Ϊ
����2molCOȼ�շų�������Ϊ![]() ��COȼ�յ��Ȼ�ѧ����ʽΪ��
��COȼ�յ��Ȼ�ѧ����ʽΪ��![]()
![]() ���W��Ԫ������������Ӧ��ˮ�����Ϊ
���W��Ԫ������������Ӧ��ˮ�����Ϊ![]() ����ת����ϵ��֪��ΪFe��C����ת����ϵ������ΪFe������Ϊ����������Ϊ��������������ΪC������Ϊ������̼����Ϊһ����̼���ʶ��ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ��3Fe+4H2O
����ת����ϵ��֪��ΪFe��C����ת����ϵ������ΪFe������Ϊ����������Ϊ��������������ΪC������Ϊ������̼����Ϊһ����̼���ʶ��ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ��3Fe+4H2O![]() Fe3O4+4H2��C+H2O
Fe3O4+4H2��C+H2O![]() CO+H2��
CO+H2��
�ʴ�Ϊ��3Fe+4H2O![]() Fe3O4+4H2��C+H2O
Fe3O4+4H2��C+H2O![]() CO+H2��
CO+H2��
![]() ���W��Ԫ�صļ��⻯��Ϊ
���W��Ԫ�صļ��⻯��Ϊ![]() ��
��![]() ��
��![]() ����������
�����γ������![]() ��������ˮ��
��������ˮ��![]() ��������Թ���һ��ȼ�ϵ�أ���������������Ӧ��
��������Թ���һ��ȼ�ϵ�أ���������������Ӧ��![]() �ڸ����ŵ磬��������������
�ڸ����ŵ磬��������������![]() ��
��![]() ����ⷴӦʽΪ��
����ⷴӦʽΪ��![]() ��
��

 �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�