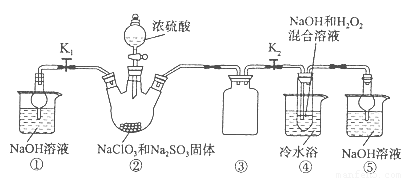
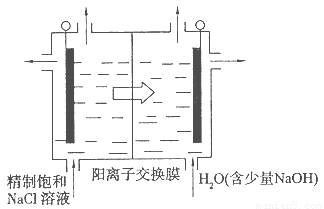
��Ŀ����
��ʯī���缫������Ȼ�ͭ���Ȼ��ƵĻ����Һ�����κ�����£�����������������ͬʱ�����ķ�Ӧ��
[ ]
A��������2H++2e- = H2�� ������4OH- - 4e- = 2H2O + O2��
B��������2H++2e- = H2�� ������2Cl- - 2e- = Cl2��
C��������Cu2++2e- = Cu ������4OH- - 4e- = 2H2O + O2��
D��������Cu2++2e- = Cu ������2Cl- - 2e- = Cl2��
B��������2H++2e- = H2�� ������2Cl- - 2e- = Cl2��
C��������Cu2++2e- = Cu ������4OH- - 4e- = 2H2O + O2��
D��������Cu2++2e- = Cu ������2Cl- - 2e- = Cl2��
C

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ
 ���CuCl2��Һ��װ����ͼ���������йص��ж���ȷ���ǣ�������
���CuCl2��Һ��װ����ͼ���������йص��ж���ȷ���ǣ������� ��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��