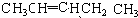��Ŀ����
�л���AΪ����������ͼ��������Է�������Ϊ70������ط�Ӧ������ʾ������B��D��E�Ľṹ�о�����2 ��-CH3��������4����ԭ�ӡ�
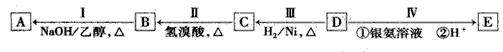
��ش��������⣺
(1)D�ķ���ʽΪ____ ��
(2)B�����������ŵ�����Ϊ___��
(3)��ķ�Ӧ����Ϊ____������ĸ��ţ���
a����ԭ��Ӧ
b���ӳɷ�Ӧ
c��������Ӧ
d����ȥ��Ӧ
(4)д�����з�Ӧ�Ļ�ѧ����ʽ�� I��____�� C��E����һ�������·�Ӧ����F��FΪ����ζ���л�������÷�Ӧ�Ļ�ѧ����ʽΪ____.
(5)A��������____��
(6)E�ж���ͬ���칹�壬����һ��ͬ���칹���ܷ���������Ӧ����������Ʒ�Ӧ�������������ܷ�����ȥ��Ӧ����ṹ��ʽΪ____��
(1) C5H10O
(2)��ԭ��
(3)a��b
(4)

(5)3-��-1-��ϩ
(6)
(2)��ԭ��
(3)a��b
(4)


(5)3-��-1-��ϩ
(6)


��ϰ��ϵ�д�
�����Ŀ