��Ŀ����
ũ��������������Ҫ���⡢������ˮ������Ҫ���ֻ�ѧԪ�أ���ֲ��ȱ��NԪ��ʱ������Ϊֲ������������Ҷɫ���ƣ�����ʱҶƬ����ֱ��������
��1�����п������ʵĻ�������______�����к�N����ߵ���______��
A������� B���������� C������� D������
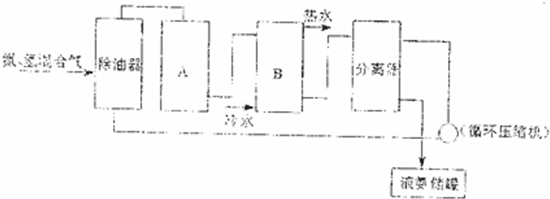
��2�����ʵ��Ʊ��������漰���ĺϳɣ�����д���кϳɰ���������ͼ�е��豸���ƣ�

A______B______
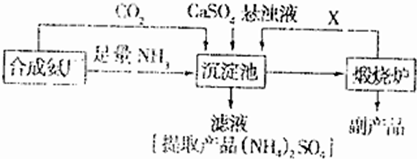
��3��ij������Ϊ���ۺ��������������еĸ���ƷCaSO4�������ڵĺϳɰ�����������������Ʊ���NH4��2SO4�Ĺ������̣�

�ٳ������з�������Ҫ��Ӧ����ʽ��______��
�����������̵ij�������ͨ������������Ŀ����______������ѭ��ʹ�õ�X��______��
�۴����ʵ����ʺ�ҵ����ʵ�ʵĽǶȿ��Ǹ����̵���Ҫȱ����______��
�⣺��1�����������ʵ�����Ӧ���е�Ԫ�أ���Ϊ����狀����أ��������صĺ�������ߣ��ʴ�Ϊ��AD��D��
��2����ҵ�ϳɰ�Ӧ�ڸ��¡���ѹ�������������½��У���Ӧ��ѹ���������������ѹǿ��ͨ���Ƚ�������������м��ȣ��ʴ�Ϊ��ѹ�������Ƚ�������
��3���ٸ��ݷ�Ӧ���̿�֪��Ӧ��ΪCaSO4��CO2��NH3��H2O��������ΪCaCO3�ͣ�NH4��2SO4����Ӧ�ķ���ʽΪCaSO4+CO2+2NH3+H2O=CaCO3��+��NH4��2SO4��
�ʴ�Ϊ��CaSO4+CO2+2NH3+H2O=CaCO3��+��NH4��2SO4��
����Һ�ʼ���������CO2�����գ�����ͨ���������������������жϿ�֪ѭ��������X��CO2��
�ʴ�Ϊ��һ�����ṩ��Ӧ���һ����ʹ��Һ�ʼ���������CO2�����գ�CO2��
��CaSO4�ܽ��С������ˮ����ҺŨ�Ƚϵͣ�����������������ᵼ�²��ʽϵͣ�
�ʴ�Ϊ������CaSO4�ܽ��С���˷�Ӧ�IJ��ʱȽϵͣ�
��������1������NԪ�ص����ʿ��������ʣ�һ����˵���صĺ�������ߣ�
��2����ҵ�ϳɰ�Ӧ�ڸ��¡���ѹ�������������½��У����ݷ�Ӧ�������жϣ�
��3�����ݷ�ӦCaSO4+CO2+2NH3+H2O=CaCO3��+��NH4��2SO4��ƽ��ԭ����֪�����ɵ�CaCO3�ܽ��С��CaSO4�������ڷ�Ӧ������У���Һ�ʼ���������CO2�����գ�����ͨ���������������������жϿ�֪ѭ��������X��CO2������Ʒ����ʯ�ң�
���������⿼�鰱�ĺϳɣ���Ŀ�ѶȲ���ע����ճ�����ҵ�Ʊ�ԭ�����ر���������ʵ����ʣ���ѧϰ��һ��Ҫ�ι̰��գ�
��2����ҵ�ϳɰ�Ӧ�ڸ��¡���ѹ�������������½��У���Ӧ��ѹ���������������ѹǿ��ͨ���Ƚ�������������м��ȣ��ʴ�Ϊ��ѹ�������Ƚ�������
��3���ٸ��ݷ�Ӧ���̿�֪��Ӧ��ΪCaSO4��CO2��NH3��H2O��������ΪCaCO3�ͣ�NH4��2SO4����Ӧ�ķ���ʽΪCaSO4+CO2+2NH3+H2O=CaCO3��+��NH4��2SO4��
�ʴ�Ϊ��CaSO4+CO2+2NH3+H2O=CaCO3��+��NH4��2SO4��
����Һ�ʼ���������CO2�����գ�����ͨ���������������������жϿ�֪ѭ��������X��CO2��
�ʴ�Ϊ��һ�����ṩ��Ӧ���һ����ʹ��Һ�ʼ���������CO2�����գ�CO2��
��CaSO4�ܽ��С������ˮ����ҺŨ�Ƚϵͣ�����������������ᵼ�²��ʽϵͣ�
�ʴ�Ϊ������CaSO4�ܽ��С���˷�Ӧ�IJ��ʱȽϵͣ�
��������1������NԪ�ص����ʿ��������ʣ�һ����˵���صĺ�������ߣ�
��2����ҵ�ϳɰ�Ӧ�ڸ��¡���ѹ�������������½��У����ݷ�Ӧ�������жϣ�
��3�����ݷ�ӦCaSO4+CO2+2NH3+H2O=CaCO3��+��NH4��2SO4��ƽ��ԭ����֪�����ɵ�CaCO3�ܽ��С��CaSO4�������ڷ�Ӧ������У���Һ�ʼ���������CO2�����գ�����ͨ���������������������жϿ�֪ѭ��������X��CO2������Ʒ����ʯ�ң�
���������⿼�鰱�ĺϳɣ���Ŀ�ѶȲ���ע����ճ�����ҵ�Ʊ�ԭ�����ر���������ʵ����ʣ���ѧϰ��һ��Ҫ�ι̰��գ�

��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
�����Ŀ
ũ��������������Ҫ���⡢������ˮ������Ҫ���ֻ�ѧԪ�ء���ֲ��ȱ��NԪ��ʱ������Ϊֲ������������Ҷɫ���ƣ�����ʱҶƬ����ֱ��������
��1�����п������ʵĻ������� �����к�N����ߵ��� ��
| A������� | B���������� | C������� | D������ |


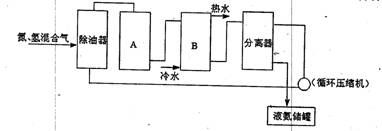
A B
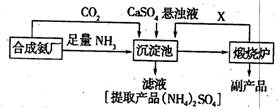
��3��ij������Ϊ���ۺ��������������еĸ���ƷCaSO4�������ڵĺϳɰ�����������������Ʊ���NH4��2SO4�Ĺ������̣�

�ٳ������з�������Ҫ��Ӧ����ʽ�� ��
�����������̵ij�������ͨ������������Ŀ���� ������ѭ��ʹ�õ�X�� ��
�۴����ʵ����ʺ�ҵ����ʵ�ʵĽǶȿ��Ǹ����̵���Ҫȱ���� ��