��Ŀ����
ˮ�ĵ���ƽ����������ͼ��ʾ��
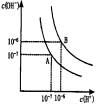
��1������A���ʾ25��ʱˮ�ڵ���ƽ��ʱ������Ũ�ȣ����¶�����100��ʱ��ˮ�ĵ���ƽ��״̬��B�㣬���ʱˮ�����ӻ���________���ӵ�________��
��2���������µ�PH��8��Ba��OH��2��Һ�볣���µ�PH��5��ϡ�����ϣ�������100��ĺ��£���ʹ�����ҺPH��7��
��Ba��OH��2������������Ϊ________��
��3����֪AnBm�����ӻ���[c��Am����]��[c��Bn����]m��ʽ��[c��Am����]n��c[��bn����]m��ʾ���ӵ����ʵ���Ũ�ȡ���ij�¶��£�Ca��OH��2���ܽ��Ϊ0.74g��
�䱥����Һ�ܶ���Ϊ1g��mL��1�������ӻ�Ϊ________��
������
��1��10��14 10��12 ��2��2��9 ��3��0.004
|

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�I���跴Ӧ��Fe(s)+CO2(g)  FeO(s)+CO(g)��ƽ�ⳣ��ΪK1��
FeO(s)+CO(g)��ƽ�ⳣ��ΪK1��
��Ӧ��Fe(s)+H2O(g)  FeO(s)+H2(g)��ƽ�ⳣ��ΪK2��
FeO(s)+H2(g)��ƽ�ⳣ��ΪK2��
�ڲ�ͬ�¶��£�K1��K2��ֵ���£�
|
T(K) |
K1 |
K2 |
|
973 |
1.47 |
2.36 |
|
1173 |
2.15 |
1.67 |
(1)���з�Ӧ��CO2(g)+H2(g)
 CO(g)+H2O(g)������һ�� (������š�)�ȷ�Ӧ��Ҫʹƽ��������ƶ����ɲ�ȡ�Ĵ�ʩ��
(�����)��
CO(g)+H2O(g)������һ�� (������š�)�ȷ�Ӧ��Ҫʹƽ��������ƶ����ɲ�ȡ�Ĵ�ʩ��
(�����)��
A����С��Ӧ�����ݻ�
B������Ӧ�����ݻ�
C�������¶�
D�������¶�
E��ʹ�ú��ʵĴ���
F���跨����CO����
(2)����ӦFe(s)+CO2(g)
 FeO(s)+CO(g)���¶�T1�½��У�Fe(s)+H2O(g)
FeO(s)+CO(g)���¶�T1�½��У�Fe(s)+H2O(g)
 FeO(s)+H2(g)���¶�T2�½��У���֪T1>T2����c(CO2)>c(H2O)(������������ͬ)�������ߵķ�Ӧ����
(�����)
FeO(s)+H2(g)���¶�T2�½��У���֪T1>T2����c(CO2)>c(H2O)(������������ͬ)�������ߵķ�Ӧ����
(�����)
A��ǰ�ߴ� B�����ߴ� C��һ���� D�����ж�
II��(1)ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȡ���100��ʱ1 mol��L��1��NaOH��Һ�У���ˮ�������c(H��)��________mol��L��1��KW(25��)________KW(100��)(�>������<������)��25 ��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4HCO3���壬��ˮ�ĵ���ƽ���Ӱ����________(��ٽ����������ơ���Ӱ�족)��
(2)����ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ����������֪������ݡ�
|
��ѧʽ |
����ƽ�ⳣ��(25��) |
|
HCN |
K��4.9��10��10 |
|
CH3COOH |
K��1.8��10��5 |
|
H2CO3 |
K1��4.3��10��7��K2��5.6��10��11 |
��25��ʱ���е�Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һ��������Һ��pH�ɴ�С��˳��Ϊ ____��
��25 ��ʱ����ͬŨ�ȡ���ͬ�����CH3COOH��Һ��NaOH��Һ��ϣ� ������Һ��c(Na��)________c(CH3COO��)(�>������<������)
����NaCN��Һ��ͨ������CO2����������Ӧ�Ļ�ѧ����ʽΪ_______________________________��
I���跴Ӧ��Fe(s)+CO2(g) ![]() FeO(s)+CO(g)��ƽ�ⳣ��ΪK1��
FeO(s)+CO(g)��ƽ�ⳣ��ΪK1��
��Ӧ��Fe(s)+H2O(g) ![]() FeO(s)+H2(g)��ƽ�ⳣ��ΪK2��
FeO(s)+H2(g)��ƽ�ⳣ��ΪK2��
�ڲ�ͬ�¶��£�K1��K2��ֵ���£�
| T(K) | K1 | K2 |
| 973 | 1.47 | 2.36 |
| 1173 | 2.15 | 1.67 |
(1)���з�Ӧ��CO2(g)+H2(g) ![]() CO(g)+H2O(g)������һ�� (������š�)�ȷ�Ӧ��Ҫʹƽ��������ƶ����ɲ�ȡ�Ĵ�ʩ�� (�����)��
CO(g)+H2O(g)������һ�� (������š�)�ȷ�Ӧ��Ҫʹƽ��������ƶ����ɲ�ȡ�Ĵ�ʩ�� (�����)��
A����С��Ӧ�����ݻ� B������Ӧ�����ݻ� C�������¶�
D�������¶� E��ʹ�ú��ʵĴ��� F���跨����CO����
(2)����ӦFe(s)+CO2(g) ![]() FeO(s)+CO(g)���¶�T1�½��У�Fe(s)+H2O(g)
FeO(s)+CO(g)���¶�T1�½��У�Fe(s)+H2O(g) ![]() FeO(s)+H2(g)���¶�T2�½��У���֪T1>T2����c(CO2)>c(H2O)(������������ͬ)�������ߵķ�Ӧ����
FeO(s)+H2(g)���¶�T2�½��У���֪T1>T2����c(CO2)>c(H2O)(������������ͬ)�������ߵķ�Ӧ����
(�����)
A��ǰ�ߴ� B�����ߴ� C��һ���� D�����ж�
II��(1)ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȡ���100��ʱ1 mol��L��1��NaOH��Һ�У���ˮ�������c(H��)��________mol��L��1��KW(25��)________KW(100��)(�>������<������)��25 ��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4HCO3���壬��ˮ�ĵ���ƽ���Ӱ����________(��ٽ����������ơ���Ӱ�족)��

(2)����ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ����������֪������ݡ�
| ��ѧʽ | ����ƽ�ⳣ��(25��) |
| HCN | K��4.9��10��10 |
| CH3COOH | K��1.8��10��5 |
| H2CO3 | K1��4.3��10��7��K2��5.6��10��11 |
��25��ʱ���е�Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һ��������Һ��pH�ɴ�С��˳��Ϊ_______ ____��
��25 ��ʱ����ͬŨ�ȡ���ͬ�����CH3COOH��Һ��NaOH��Һ��ϣ�
������Һ��c(Na��)________c(CH3COO��)(�>������<������)
����NaCN��Һ��ͨ������CO2����������Ӧ�Ļ�ѧ����ʽΪ
___________________________________________________________________��
 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��

