��Ŀ����
ij�о���ѧ��С��������ϵ�֪��Ư����������Һ��Ӧ����ȡ��������ѧ����ʽΪ��
Ca(ClO)2��CaCl2��2H2SO4==2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣
Ca(ClO)2��CaCl2��2H2SO4==2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣

�Իش�
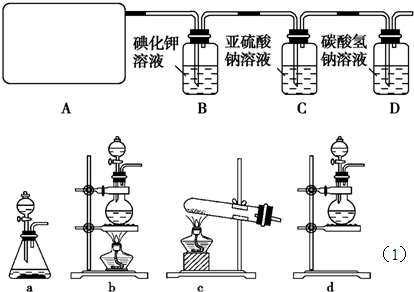
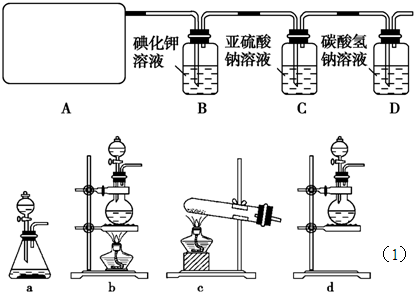
��1����ʵ����A���ֵ�װ����________(��дװ�õ����)��
��1����ʵ����A���ֵ�װ����________(��дװ�õ����)��

�Իش�
��2����ʵ����A���ֵ�װ����________(��дװ�õ����)��
��3��B�з�Ӧ�Ļ�ѧ����ʽ��______________��
��4��д��C�з�Ӧ�����ӷ���ʽ______________����������С��ͬѧ���һ��ʵ��,֤��ϴ��ƿC�е�Na2SO3�ѱ�����(����ʵ�鲽��)��________________��
��4��д����Dװ���з�����Ӧ�����ӷ���ʽ____________________��
��5����ʵ��������Ե�ȱ����__________________________________��
��6����С���ֽ���������ʵ�飺��ȡƯ��2.0 g����ĥ���ܽ⣬���Ƴ�250 mL��Һ�����������KI��Һ������H2SO4��Һ�����á�����ȫ��Ӧ����0.1 mol/L��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ2Na2S2O3��I2===Na2S4O6��2NaI����Ӧ���ʱ��������Na2S2O3 200 mL�����Ư����Ca(ClO)2����������Ϊ_______��
��2����ʵ����A���ֵ�װ����________(��дװ�õ����)��
��3��B�з�Ӧ�Ļ�ѧ����ʽ��______________��
��4��д��C�з�Ӧ�����ӷ���ʽ______________����������С��ͬѧ���һ��ʵ��,֤��ϴ��ƿC�е�Na2SO3�ѱ�����(����ʵ�鲽��)��________________��
��4��д����Dװ���з�����Ӧ�����ӷ���ʽ____________________��
��5����ʵ��������Ե�ȱ����__________________________________��
��6����С���ֽ���������ʵ�飺��ȡƯ��2.0 g����ĥ���ܽ⣬���Ƴ�250 mL��Һ�����������KI��Һ������H2SO4��Һ�����á�����ȫ��Ӧ����0.1 mol/L��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ2Na2S2O3��I2===Na2S4O6��2NaI����Ӧ���ʱ��������Na2S2O3 200 mL�����Ư����Ca(ClO)2����������Ϊ_______��
��1��b
��2��Cl2+2KI=2KCl+I2
��3��Cl2+SO32-+H2O=SO42-+2Cl-+2H+��ȡ������Ӧ�����Һ���Թ��У�����HCl��Һ�����ٲ�������Ϊֹ���ٵμ�BaCl2��Һ������а�ɫ�������ɣ�֤��Na2SO3�ѱ�����
��4��Cl2+H2O H++Cl-+HClO��HCO3-+H+=H2O+CO2��������Cl2+ HCO3-=CO2��+Cl-+HClO)
H++Cl-+HClO��HCO3-+H+=H2O+CO2��������Cl2+ HCO3-=CO2��+Cl-+HClO)
��5����β������װ��
��6��35.8%(��35.75%)
��2��Cl2+2KI=2KCl+I2
��3��Cl2+SO32-+H2O=SO42-+2Cl-+2H+��ȡ������Ӧ�����Һ���Թ��У�����HCl��Һ�����ٲ�������Ϊֹ���ٵμ�BaCl2��Һ������а�ɫ�������ɣ�֤��Na2SO3�ѱ�����
��4��Cl2+H2O
 H++Cl-+HClO��HCO3-+H+=H2O+CO2��������Cl2+ HCO3-=CO2��+Cl-+HClO)
H++Cl-+HClO��HCO3-+H+=H2O+CO2��������Cl2+ HCO3-=CO2��+Cl-+HClO)��5����β������װ��
��6��35.8%(��35.75%)

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 l2��2H2SO4
l2��2H2SO4 2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣
2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣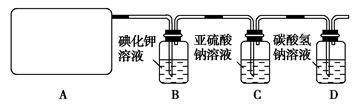

 ________________ ��
________________ ��
