��Ŀ����
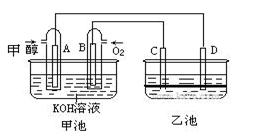
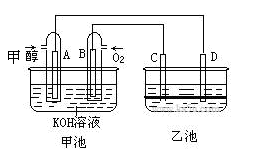
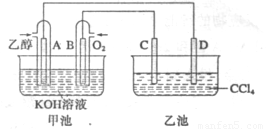
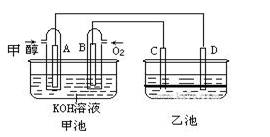
��9�֣���ͼ�׳غ��ҳ��е��ĸ��缫���Ƕ��Բ��ϣ��ҳ���Һ�ֲ㣬�ϲ���ҺΪ����Һ�������ԣ������ͼʾ�ش��������⣺
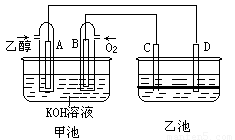
(1)ͨ���Ҵ���C2H5OH���Ķ��Ե缫�ĵ缫��ӦʽΪ ��
���׳ؿ��Գ�磬���ʱA�ӵ�Դ�ĸ�������ʱB�������ĵ缫��ӦʽΪ ��
(2)���ҳط�Ӧ�����У����Թ۲쵽 �缫��Χ����Һ�����غ�ɫ����Ӧ��Ϻ��ò�����������Һ�����²���Һ�����Ϻ�ɫ���ϲ�ӽ���ɫ��C�������ĵ缫��Ӧʽ ��
(3)���ڳ��³�ѹ�£�1gC2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29.71kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
(1)C2H5OH+16OH--12e-=2CO32-+11H2O (2��) 4OH--4e-=2H2O+O2�� (2��)
(2)C (1��) 2I--2e‑=I2 (2��)
(3)C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l)����H=-1366.7kJ��mol (2��)
����������1���׳�Ϊ�Ҵ�ȼ�ϵ�أ�C2H5OH��3O2=2CO2��3H2O��CO2��2OH��=CO32����H2O
�ܷ�ӦΪ��C2H5OH��3O2 ��4OH��=2CO32����5H2O
������C2H5OH+16OH--12e-=2CO32-+11H2O ������2H2O+O2 +4e-=4OH-
������ʱ��������2CO32-+11H2O+12e-=C2H5OH+16OH- ������4OH--4e-=2H2O+O2��
��2��������֪���ϲ�����ҺӦ���ǵ⻯��������ɵ��ʵ⣬�������ܶȴ�Сˮ��Һ���²�Һ���У������Ϻ�ɫ��C����ԭ���������Ϊ������2I--2e‑=I2
��3���������1mol�Ҵ���ȫȼ�տɷų�����Ϊ1366.7kJ

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�