��Ŀ����
ij��ѧ����С����ʵ�������������ͼ��ʾ��ʵ��װ�ã����С����Ĵ�������ʵ�顣

��1��A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ�����______________���ѧʽ����ͼ�пհ״���������ӦΪ____________________��ѡ������������ţ��̶�װ��ʡ�ԣ���

��2����װ�ò�����������Ȼ����һ��ȱ�ݣ��ԴӰ�ȫ�뻷���ĽǶ������ǣ��Ը�װ�ý��иĽ���__________________________�� ��________________________________��
��3�����ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B������_______________________________________��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ��______________________________��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ��������_______________��
��3�����ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B������_______________________________________��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ��______________________________��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ��������_______________��
��1�� NH4HCO3��(NH4)2CO3��e��f��g��h
��2������װ��C��D֮������һ��������װ�ã�����D������һ��β������װ��
��3��������CO2��ˮ������������O2����4NH3+5O2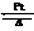 4NO+6H2O����ͭƬ����ֱ���ܽ⣬��Һ����ɫ��������ɫ���岢�ڹ��ƿ�Ϸ���ɺ���ɫ
4NO+6H2O����ͭƬ����ֱ���ܽ⣬��Һ����ɫ��������ɫ���岢�ڹ��ƿ�Ϸ���ɺ���ɫ
��2������װ��C��D֮������һ��������װ�ã�����D������һ��β������װ��
��3��������CO2��ˮ������������O2����4NH3+5O2
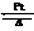 4NO+6H2O����ͭƬ����ֱ���ܽ⣬��Һ����ɫ��������ɫ���岢�ڹ��ƿ�Ϸ���ɺ���ɫ
4NO+6H2O����ͭƬ����ֱ���ܽ⣬��Һ����ɫ��������ɫ���岢�ڹ��ƿ�Ϸ���ɺ���ɫ 
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ








