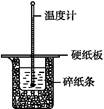
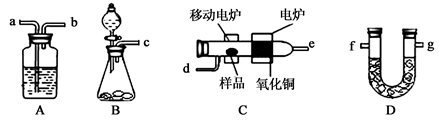
��Ŀ����
����˵����ȷ����
��H��0����v(SO2)= v(SO3)ʱ��˵���÷�Ӧ�Ѵﵽƽ��״̬
| A�������£����ʵ���Ũ�Ⱦ�Ϊ0.1mol��L?1Na2CO3��NaHCO3�ĵ���������Һ�У� 2c(OH?)��2c(H+)��3c(H2CO3)��c(HCO3��)��c(CO32?) |
| B����H��0,��S��0�ķ�Ӧ�����Է���Ӧ����H��0,��S��0�ķ�Ӧ�κ��������Ƿ��Է���Ӧ�� |
| C����֪��P4(g)��6Cl2(g)��4PCl3(g) ��H��akJ��mol��1 P4(g)��10Cl2(g)��4PCl5(g)��H��bkJ��mol��1 P4������������ṹ��PCl5��P��Cl���ļ���ΪckJ��mol��1,PCl3��P��Cl���ļ���Ϊ1.2ckJ��mol��1���ɴ˼���Cl��Cl���ļ��� 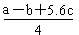 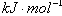 |
D����һ���¶��£��̶����Ϊ2L�ܱ������У�������Ӧ��2SO2(g)+O2(g) 2SO3(g) 2SO3(g) |
A
���������A�����������غ㣬����Na2CO3��Һ��c(OH?)=c(H+)+2c(H2CO3)��c(HCO3��)������NaHCO3��Һ��c(OH?)+c(CO32?)��c(H+)+c(H2CO3)�����ߵ�Ũ�ȡ��������Ϻ���Һ���������ʵ����ʵ�����ȣ����ԣ���������ʽ���ӣ�[c(OH?)=c(H+)+2c(H2CO3)��c(HCO3��)]+[ c(OH?)+c(CO32?)��c(H+)+c(H2CO3)]���������2c(OH?)��2c(H+)��3c(H2CO3)��c(HCO3��)��c(CO32?)����ȷ��B����H��0����S��0 �ڽϸ��¶������Է�������C����������Ӧʽ�ֱ���Ϊ�ٺ͢ڣ�(�� - ��)��4�ɵã�PCl3(g)+Cl2(g)= PCl5(g) ����H��
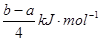 ���ɣ�
���ɣ�PCl3(g) + Cl2(g) = PCl5(g)
��1��P��Cl�� ��1��Cl��Cl�� ��1��P��Cl��
3��1.2c Q 5��c
(3.6c + Q -5c)
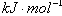 =
=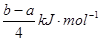 �� Q=
�� Q=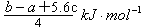 ����C����
����C����Dѡ���ͬ���ʱ�ʾ���ʣ�����ƽ��ʱ����������֮�ȵ��ڻ�ѧ������֮�ȣ��磬V����SO2����V�棨SO3��=" 2" : 2����V����SO2��=V�棨SO3��������v(SO2)=v(SO3)δָ����Ӧ���ʵķ��ʴ���

��ϰ��ϵ�д�
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
�����Ŀ


 CO(g)��3H2(g)���÷�Ӧ��H����206 kJ��mol��1��
CO(g)��3H2(g)���÷�Ӧ��H����206 kJ��mol��1�� O2(g)
O2(g)  SO3(g)��H����98.3 kJ��mol��1
SO3(g)��H����98.3 kJ��mol��1 O2(g)
O2(g) 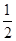 H2(g)��
H2(g)�� H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣
H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣