��Ŀ����
����Ŀ����������һ����������Ѫ�ܼ�����ҩ����ɻ�����![]() ��
��![]() ��һ�������ºϳɵõ������ַ�Ӧ�����ԣ���
��һ�������ºϳɵõ������ַ�Ӧ�����ԣ���

��ش��������⣺
��1��![]() ������Ϊ______��
������Ϊ______��![]() �ķ�Ӧ����Ϊ______��
�ķ�Ӧ����Ϊ______��
��2��![]() �ķ���ʽ��______��
�ķ���ʽ��______��![]() �ķ�Ӧ�У�����Ļ�����
�ķ�Ӧ�У�����Ļ�����![]() ��������Һ�ɷ���������Ӧ����������Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��������Һ�ɷ���������Ӧ����������Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��3��![]() Ϊȡ����Ӧ������һ��������еĹ�����������____________��
Ϊȡ����Ӧ������һ��������еĹ�����������____________��![]() ��ȫȼ��������Ҫ���� ____________
��ȫȼ��������Ҫ���� ____________![]() ��
��
��4��![]() ��ͬ���칹��
��ͬ���칹��![]() �Ƿ����ᣬ
�Ƿ����ᣬ ��
��![]() �ĺ˴Ź�������ֻ������壬
�ĺ˴Ź�������ֻ������壬![]() �Ľṹ��ʽΪ____________��
�Ľṹ��ʽΪ____________��![]() �Ļ�ѧ����ʽΪ________________________��
�Ļ�ѧ����ʽΪ________________________��
��5����ͼ�У������ϳ����߷��ӻ�����ķ������____________ ��д���ܼ�������ʴ��ڵ���ɫ��Ӧ�����õ��Լ���ʵ������__________________________________________��
��6����֪��![]() ��ԭ��Ϊ����C6H5OH+
��ԭ��Ϊ����C6H5OH+ +C2H5OH����
+C2H5OH����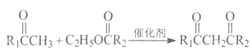 +C2H5OH��
+C2H5OH��![]() �Ľṹ��ʽΪ____________��
�Ľṹ��ʽΪ____________��
���𰸡���Ȳ �ӳɷ�Ӧ C10H10O ![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH![]()
![]() +2Ag��+3NH3+H2O �Ȼ� 4
+2Ag��+3NH3+H2O �Ȼ� 4 ![]()
![]() +2NaOH
+2NaOH![]() +NaCl+H2O ���� �Ȼ�����Һ����Һ����ɫ
+NaCl+H2O ���� �Ȼ�����Һ����Һ����ɫ 
��������
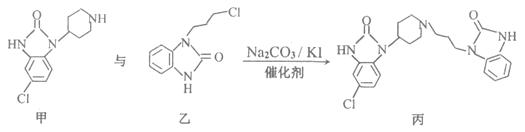
������X��������Һ�ɷ���������Ӧ��˵��X�к���ȩ�������D��E�Ľṹ��ʽ��֪��XΪ����ȩ������L��M�ķ�Ӧԭ����֪��L��M��ȡ����Ӧ���ɢٵķ�Ӧ��Ϣ��L�з��ǻ���C2H5OCOOC2H5��Ӧ��-COOC2H5����ǻ�Hԭ�ӽ������ɢڵķ�Ӧ��Ϣ��֪������������������M����M�Ľṹ��ʽΪ ��������E��M��һ�������ºϳɵõ������֣��ݴ˷������
��������E��M��һ�������ºϳɵõ������֣��ݴ˷������
(1)A�Ľṹ��ʽΪCH3C��CH������̼̼����������Ȳ��������Ϊ��Ȳ��A��B�DZ�Ȳ��H2O��Ӧ����Ӧ��̼̼����ת��Ϊ̼̼˫���������˼ӳɷ�Ӧ���ʴ�Ϊ����Ȳ���ӳɷ�Ӧ��
(2)�ɽṹ��ʽ ��֪��E�ķ���ʽΪC10H10O��XΪ����ȩ(
��֪��E�ķ���ʽΪC10H10O��XΪ����ȩ(![]() )������ȩ��������Һ��Ӧ�Ļ�ѧ����ʽΪ��
)������ȩ��������Һ��Ӧ�Ļ�ѧ����ʽΪ��![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH![]()
![]() +2Ag��+3NH3+H2O���ʴ�Ϊ��C10H10O��
+2Ag��+3NH3+H2O���ʴ�Ϊ��C10H10O��![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH![]()
![]() +2Ag��+3NH3+H2O��
+2Ag��+3NH3+H2O��
(3)GΪ��������G�뱽������Jͬʱ�����������ᣬ�����к��еĹ��������Ȼ���G�ķ���ʽΪC4H6O3��G��ȫȼ�����ɶ�����̼��ˮ��1molG��ȫȼ�գ����ĵ����������ʵ���Ϊ(4+![]() -
-![]() )mol=4mol���ʴ�Ϊ���Ȼ���4��
)mol=4mol���ʴ�Ϊ���Ȼ���4��
(4)L�ķ���ʽ��C8H8O��Q��L��ͬ���칹�壬Q���ڷ����ᣬQ�к��Ȼ���Q��R�DZ����ϵļ��ϵ�1��Hԭ�ӱ�ȡ����R��S���ȴ�����ˮ�ⷴӦ��S��T��-CH2OH������-COOH��T�ĺ˴Ź�������ֻ������壬˵��2���Ȼ����ڱ����Ķ�λ��QΪ�Լ������ᣬQ�ṹ��ʽΪ![]() ��RΪ
��RΪ![]() ����R��S�Ļ�ѧ����ʽΪ��
����R��S�Ļ�ѧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
(5)���ӿ����ȩ���ϳ����߷��ӻ����������ǻ����ɼ����Ȼ�����Һ����Һ����ɫ���ʴ�Ϊ�����ӣ��Ȼ�����Һ����Һ����ɫ��
(6)L��M��ȡ����Ӧ���ɢٵķ�Ӧ��Ϣ��L�еķ��ǻ���C2H5OCOOC2H5��Ӧ��-COOC2H5����ǻ�Hԭ�ӽ������ɢڵķ�Ӧ��Ϣ��֪������������������M����M�Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

����Ŀ����.ijѧϰС���á���ӵ��������ⶨ����CuCl2��2H2O�������������������I-������Ӧ�������������ʣ��Ĵ��ȣ��������£�ȡ0.800g ��������ˮ���������KI ���壬��ַ�Ӧ�����ɰ�ɫ��������0.1000 mol/L Na2S2O3 ����Һ�ζ�������ζ��յ�ʱ��ƽ������Na2S2O3 ����Һ40.00 mL������֪��2Cu2++4I-=2CuI��+I2��I2+2S2O32-=S4O62-+2I-����
��1����ѡ��________���ζ�ָʾ�����ζ��յ��������________________��
��2����������CuCl2��2H2O �������ٷ���Ϊ________________��
��.25�棬������ĵ���ƽ�ⳣ���������Ҫ��ش��������⡣
K1 | K2 | |
H2SO3 | 1.3��10-2 | 6.3��10-8 |
H2CO3 | 4.2��10-7 | 5.6��10-11 |
��1�������ӷ���ʽ��ʾ����������Һ�ʼ��Ե�ԭ��________________________________��
��2��Ũ�Ⱦ�Ϊ0.1mol/L��Na2SO3��Na2CO3����Һ��SO32-��CO32-��HSO3-��HCO3-Ũ�ȴӴ�С��˳��Ϊ________________________________��
��3������������Һ��ͨ������CO2����Ļ�ѧ����ʽ________________________________��
����Ŀ���������������ǵij��м���������˼����Σ�����о�![]() ��
��![]() ��
��![]() �ȴ�����Ⱦ����Ĵ���������Ҫ���塣
�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1�������Ƽ�ѭ�������ѳ������е�![]() ��
��
�����Ƽ�ѭ�����У�![]() ��Һ��Ϊ����Һ������
��Һ��Ϊ����Һ������![]() ��Һ����
��Һ����![]() �Ƶã��÷�Ӧ�����ӷ���ʽ��____��
�Ƶã��÷�Ӧ�����ӷ���ʽ��____��
������Һ����![]() �Ĺ����У�
�Ĺ����У�![]() ��
��![]() �仯��ϵ���±���
�仯��ϵ���±���
|
| 1��1 |
|
|
|
|
|
���ϱ��жϣ�![]() ��Һ��______�ԣ������������������������������û�ѧƽ��ԭ�����ͣ�______��
��Һ��______�ԣ������������������������������û�ѧƽ��ԭ�����ͣ�______��
�۵�����Һ��![]() ����ԼΪ6ʱ����������������������ʾ��ͼ���£�
����ԼΪ6ʱ����������������������ʾ��ͼ���£�

д��![]() �������ŵ�ĵ缫��Ӧʽ��________________��������������Һ
�������ŵ�ĵ缫��Ӧʽ��________________��������������Һ![]() ����8����ʱ������Һ������ѭ�����á�
����8����ʱ������Һ������ѭ�����á�
��2����![]() ����ԭ
����ԭ![]() �������������������Ⱦ�����磺
�������������������Ⱦ�����磺
![]()
![]()
![]()
![]()
���ñ�״����![]() ��ԭ
��ԭ![]() ��
��![]() ������������ת�Ƶĵ�������Ϊ______�������ӵ�������ֵ��
������������ת�Ƶĵ�������Ϊ______�������ӵ�������ֵ��![]() ��ʾ�����ų�������Ϊ______
��ʾ�����ų�������Ϊ______![]() ��
��
��3����ҵ�Ϻϳɰ������������Ʊ������У����е�һ����ӦΪ��
![]()
![]()
һ�������£���![]() ��
��![]() �������Ϊ
�������Ϊ![]() �����ܱ������з���������Ӧ���ﵽƽ��ʱ���
�����ܱ������з���������Ӧ���ﵽƽ��ʱ���![]() ��
��![]() �����Ϊ1��6����ƽ�ⳣ��
�����Ϊ1��6����ƽ�ⳣ��![]() ______��
______��