��Ŀ����
����ѡ���е�������ǰ�ߴ��ں��ߵ��ǣ� ��
A����ͬ��ͬŨ�ȵ������£�NaHSO4��NaHCO3��Һ��ˮ�ĵ���̶�
B��ͬ���£�Ũ�Ⱦ�Ϊ0.1mol?L�D1��Na2S2O3��Һ��ϡ�����10������20������� ��ķ�Ӧ���ʣ�Na2S2O3+2HCl 2NaCl+S��+SO2��+H2O
C����pH=4������ʹ���ֱ�ϡ�ͳ�pH=5����Һ������ˮ����
D����������������������ͬ��������Һ��ϣ���1��NaOH+H2SO4����2��NaOH+HCl����Ϻ�������Һ��pH
D

 1��2һ����������������������Ӽ�����ͼΪʵ�����Ʊ�1��2һ���������װ��ͼ��ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ����Թ�d��װ��Һ�壮
1��2һ����������������������Ӽ�����ͼΪʵ�����Ʊ�1��2һ���������װ��ͼ��ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ����Թ�d��װ��Һ�壮��֪��CH3CH2OH
| Ũ���� |
| 170�� |
CH3CH2OH
| Ũ���� |
| 140�� |
��������б����£�
| �Ҵ� | 1��2-�������� | ���� | �� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
| �ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
| �е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
| �۵�/�� | -114.3 | 9.79 | -116.2 | -7.2 |
| ˮ���� | ���� | ���� | �� | ���� |
��2����ȫƿb��ʵ�����ж������ã���һ���Լ��ʵ������е���d�Ƿ�����������д����������ʱƿb�е��������ʵ���е���d����������Ϊ���ܵ�ԭ���ǣ���ȫƿb��������������
��3������c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������࣬���װ�õ�������û�����⣬�Է������ܵ�ԭ��
��5����ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ
��6��ʵ����Ҳ���Գ�ȥdװ����ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�õ��Թ��ڣ����ʱ��ˮ����������ȴ1��2һ��������������⣬��������������
[��ѧ����ѡ�����ʽṹ������]��30�֣�
��18�֣���ѡ�����и����з��������ѡ�
��1�� ���������У����ں��й��ۼ������Ӿ�����
A��CsCl B��KOH C��H2O D��H2
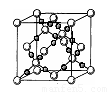
��2����֪CsCl������ܶ�Ϊ ��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ
��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ

A��  g/mol B��
g/mol B�� g/mol
g/mol
C��  g/mol D��
g/mol D�� g/mol
g/mol
��3����֪���������ͨʽXOm(OH)n����ʾ����X��S��m=2��n=2�������ʽ�Ӿͱ�ʾH2SO4��һ����ԣ���ʽ��m��ֵԽ�ú����������Խǿ�����и���������������ǿ����
A��HMnO4 B��H2SeO3 C��H3BO3 D��H3PO4
��12�֣����в���ǰ������Ԫ�ص����ʻ�ԭ�ӽṹ���±���
|
Ԫ�ر�� |
Ԫ�����ʻ�ԭ�ӽṹ |
|
A |
ԭ�ӵĵ����Ų�ͼΪ |
|
B |
�����µ���Ϊ˫ԭ�ӷ��ӣ�ԭ�Ӽ��γ����Թ��õ��Ӷ� |
|
C |
ԭ�ӵ�s�������������p�����������Ԫ�ص������Ϊ-2�� |
|
D |
������������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ |
|
E |
ԭ��������D������ |
��������������ش��������⣺(����ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��
��3��E�����ڱ��е�λ���� ��ECl3����B��C���⻯���γ���λ��Ϊ6��������������������ʵ���֮��Ϊ2��1������������λ����磬ECl3�γɵ������Ļ�ѧʽΪ ��
��4��AC2�ڸ��¸�ѹ�����γɵľ�������ͼ��ʾ���þ������������ ��ѡ����ӡ�����ԭ�ӡ��������ӡ������������壬�þ�����Aԭ�ӵ��ӻ���ʽΪ ��
��5��D �ĵ����ڿ�����ȼ�շ���ҫ�۵İ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ�� ��
���й��л���ķе㣺
�Լ� | ���� | �Ҵ� | ���� | ���� |
���� | �е�/�� | 34.5 | 78.3 | 118 |
ij����С����Ƶ�ʵ������ȡ�϶�������������װ����ͼ��ʾ�����з����Ҵ�����ˮ�����ƺ�Ũ���ᣬ���з��б���̼������Һ��������������⣺

(1)����Ũ�����������_______________������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ��______________________________��
(2)�������ιܵ�����������_______________��_______________������Ӧǰ��������Һ�μӼ��η�̪���ʺ�ɫ�����������ԭ����(�����ӷ���ʽ��ʾ)________________________����Ӧ���������е�������_________________________________________________________��
(3)��2�з���������������л�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ����˷����_______________���ټ���(�˿մ�����ѡ����ѡ��)_______________��Ȼ����������ռ�77 �����ҵ���֣��Եõ��ϴ���������������
A.���������� B.��ʯ�� C.��ˮ������ D.��ʯ��
�Լ� | ���� | �Ҵ� | ���� | �������� |
�е�(��) | 34.5 | 78.3 | 118 | 77.1 |
ij����С����Ƶ�ʵ������ȡ�϶�������������װ����ͼ��ʾ��A�з����Ҵ�����ˮ�����ƺ�Ũ���ᣬB�з��б���̼������Һ���Իش�

(1)A��Ũ�����������___________������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ��________________________________��
(2)B�����ܵ�����������___________��___________������Ӧǰ��B����Һ�μӼ��η�̪���ʺ�ɫ�������������ԭ����_____________(�����ӷ���ʽ��ʾ)����Ӧ������B�е�������_____________��
(3)��B�з���������������л�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ����˷����____________���ټ���(�˿մ�����ѡ����ѡ��)____________��Ȼ����������ռ�
A.���������� B.��ʯ�� C.��ˮ������ D.��ʯ��



