��Ŀ����
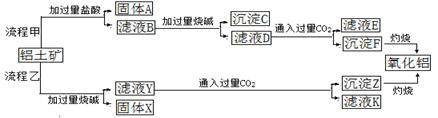
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ��Na3AlF6��������ڵ���Ƶá����������Ҫ�ɷ���Al2O3��SiO2������������NaOH��Һ�����ʡ���������������Al2O3���������£�
�ش��������⣺
��1��д����Ӧ1�Ļ�ѧ����ʽ ��
��2����Һ���м���CaO���ɵij����� ����Ӧ2�����ӷ���ʽΪ ��
��3����������Ļ�ѧ����ʽ�� ����ʯīΪ�缫�����������Ļ������ijɷ��� ��
��1��2NaOH��SiO2=Na2SiO3��H2O��2�֣� 2NaOH��Al2O3=2NaAlO2��H2O��2�֣�
��2��CaSiO3��1�֣�
2AlO2��+CO2+3H2O=2Al(OH)3��+CO32�� ��AlO2��+CO2+2H2O=Al(OH)3��+HCO3����2�֣�
��3��2Al2O3 4Al��3O2����2�֣� O2��CO2��CO����2�֣�
4Al��3O2����2�֣� O2��CO2��CO����2�֣�
���������������1��������������������ﶼ������ǿ����Һ�������κ�ˮ����2���������Ǽ����������ˮ��Ӧ�����������ƣ����������������ӽ�ϳɹ���Ƴ�����ƫ��������Һͨ�����������CO2��������ȡ����������������3�������������������ȡ����������ӦʽΪ2O2����2e��=O2����������C��O2��Ӧ����CO2��CO��
���㣺���⿼�����Ԫ�ؼ��仯�������Ҫ���ʼ�Ӧ�á�

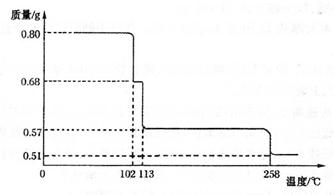
 ���ݼ���ϵ�д�
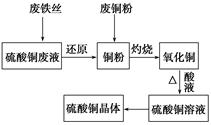
���ݼ���ϵ�д��ס�����ͬѧ��ʵ���ҷֱ�ȡ�ò�ͬ�Լ�����ȡAl(OH)3����ѡ�Լ��У�AlCl3��Һ��A12(SO4)3��Һ��NaOH��Һ����ˮ����
��1��������±���
| | ��ͬѧ | ��ͬѧ |
| ѡ���Լ� | A1C13��Һ��NaOH��Һ | A12(SO4)3��Һ����ˮ |
| ���� | ��AlCl3��Һ����μ���NaOH��Һ������ | ��A12(SO4)3��Һ����μ��백ˮ������ |
| ʵ������ | | |
| ��ѧ����ʽ | | A12(SO4)3+6NH3��H2O=2A1(OH)3 ��+3(NH4)2SO4 |
| ���ӷ���ʽ | | |
��2������������ȡAl(OH)3���Լ���ϻ������� ��
��3���ɼס�����ͬѧ��ʵ���������ܵó��Ľ�����(�ü�Ҫ����˵��) ��
ZnO���п����ԣ�Ҳ����Ҫ����̥���Ӽ�����ҵ���ɴ�ZnO(��FeO��CuO)�Ʊ�����ZnO�������£� 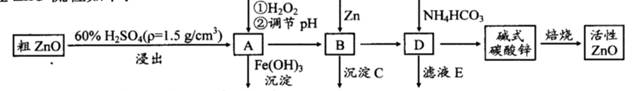
��֪����Һ��Fe2+��Fe3+��Cu2+��Zn2+�γ����������pH���±���
| ���� | ��ʼ������pH | ��ȫ������pH |
| Fe2+ | 6.4 | 8.4 |
| Fe3+ | 2.4 | 3.1 |
| Cu2+ | 5.2 | 6.7 |
| Zn2+ | 6.8 | 9 |
(1)ʵ��������98��H2SO4������100 mL60��ϡ��������ʹ�õIJ��������У��ձ�����Ͳ�� �� �� ��
(2)д����A�м�H2O2�����ӷ���ʽ�� ��
(3)��A�п��Լ��� (д��ѧʽ)������ҺpH��Χ�� ֮�䣻����CΪ ��
(4)��ʽ̼��п[Zn3(OH)4CO3��H2O]�����Ʊ�����ZnO�Ļ�ѧ����ʽΪ ��