��Ŀ����
�̲��о���������ͼװ�ý����й����ʵ��Ʊ������ʼ��飮

��1���밴Ҫ������±��ո�
| ʵ������ | ѡ�õ�����������ĸ�� | C��D����ʢ�Լ��������ƣ� | C��D�е����� |
| �������Na2CO3��NaHCO3 | BD | ����ʯ��ˮ | ______ |
| ͭ��Ũ���ᷴӦ | AC | ______ | ��Һ��ɫ��ȥ |
| �Ʊ��������� | ______ | ����̼������Һ | �ϲ�������״Һ�����������ζ |
A��Cu��Ũ���ᷴӦʱ������װ��C���Թܿ���մ�м�Һ����
B������������Cu��Ũ���ᷴӦ���������Һ���ܳ��ֺ�ɫ
C���Ʊ���������ʱ��װ��C�ĵ���ĩ����Һ������
D��Bװ�ÿ������Ʊ�������
�⣺��1��̼�����Ʋ��ȶ��������ֽ�����̼���ƺͶ�����̼���壬���ó���ʯ��ˮ���飻
ͭ��Ũ�����ڼ��������·�Ӧ���ɾ���Ư���ԵĶ����������壬����Ʒ����飻
�Ʊ������������Ҵ���������Ũ���������¼����Ʊ����ñ���̼������Һ���գ�
�ʴ�Ϊ��
��2��A��Cu��Ũ���ᷴӦ���ɶ���������Ӧ�����Թܿ���մ�м�Һ�������ɷ�ֹ��Ⱦ��������A��ȷ��
B�����������Ư���Ծ��в��ȶ��ԣ����ȿɱ�Ϊ��ɫ����B����
C��װ��C�ĵ���ĩ�˲��ܲ���Һ�����£�Ҫ��ֹ��������C����
D��ʵ���ҿ����Ȼ�狀����������Ʊ�����������Bװ�ã���D��ȷ��
�ʴ�Ϊ��AD��
��������1��̼�����Ʋ��ȶ��������ֽ�����̼���ƺͶ�����̼���壻ͭ��Ũ�����ڼ��������·�Ӧ���ɾ���Ư���ԵĶ����������壻�Ʊ������������Ҵ���������Ũ���������¼����Ʊ����ñ���̼������Һ���գ�
��2��A��Cu��Ũ���ᷴӦ���ɶ���������Ӧ��
B�����ݶ��������Ư�����жϣ�
C��Ҫ��ֹ��������
D��ʵ���ҿ����Ȼ�狀����������Ʊ�������
�����������ۺϿ�������ʵ��ķ�������ƣ���Ŀ���������ʵ��Ʊ��������Լ�������ʵ����ʵĿ��飬�ѶȲ���ע�����֪ʶ�Ļ��ۣ�
ͭ��Ũ�����ڼ��������·�Ӧ���ɾ���Ư���ԵĶ����������壬����Ʒ����飻
�Ʊ������������Ҵ���������Ũ���������¼����Ʊ����ñ���̼������Һ���գ�
�ʴ�Ϊ��
| ����ʯ��ˮ����� | |||
| Ʒ����Һ | |||
| AC |
B�����������Ư���Ծ��в��ȶ��ԣ����ȿɱ�Ϊ��ɫ����B����
C��װ��C�ĵ���ĩ�˲��ܲ���Һ�����£�Ҫ��ֹ��������C����
D��ʵ���ҿ����Ȼ�狀����������Ʊ�����������Bװ�ã���D��ȷ��
�ʴ�Ϊ��AD��
��������1��̼�����Ʋ��ȶ��������ֽ�����̼���ƺͶ�����̼���壻ͭ��Ũ�����ڼ��������·�Ӧ���ɾ���Ư���ԵĶ����������壻�Ʊ������������Ҵ���������Ũ���������¼����Ʊ����ñ���̼������Һ���գ�
��2��A��Cu��Ũ���ᷴӦ���ɶ���������Ӧ��
B�����ݶ��������Ư�����жϣ�
C��Ҫ��ֹ��������
D��ʵ���ҿ����Ȼ�狀����������Ʊ�������
�����������ۺϿ�������ʵ��ķ�������ƣ���Ŀ���������ʵ��Ʊ��������Լ�������ʵ����ʵĿ��飬�ѶȲ���ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���г�ȥ�����У����ó����Լ�����ȷ����
| ��� | ��Ҫ�ɷ� | ���� | ���ó����Լ� |
| A | MgCl2 | FeCl3 | MgO |
| B | NaOH | Na2CO3 | ���� |
| C | Fe��OH��3 | Al��OH��3 | NaOH��Һ |
| D | ��ҵ��ˮ | Hg2+ | S2- |
- A.A
- B.B
- C.C
- D.D
������ʵ������ķ�����������ȷ����
| �� �� | ʵ������ | �ᡡ �� | |
| A | ����Һ�м����Ȼ�����ϡ����� �� �� Һ | ������ɫ �� �� | ����Һ��һ������SO42-��SO32- |
| B | ����Һ������ɫ��Ӧ | ��ɫ�ʻ�ɫ | ����Һһ����ij���ε���Һ |
| C | ����Һ�м�����������ϡ����� �� �� Һ | ������ɫ �� �� | ����Һһ����ij���Ȼ������Һ |
| D | ��ʪ��ĵ⻯��-������ֽ�������ɫ�Ķ������� | ��ֽ����ɫ | ����һ���������� |
- A.A
- B.B
- C.C
- D.D
 B
B C
C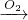 D
D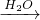 F��
F��