��Ŀ����
ij��Ӧ���У������������������̡������� ����������Ƿ�Ӧ����������ϸ������ֳ���侻��Ӧ����ʽ��ʾ��
����������Ƿ�Ӧ����������ϸ������ֳ���侻��Ӧ����ʽ��ʾ��
______+______ ______+______+______
______+______+______
��1�����������������ӷ���ʽ����ƽ����ϵ�����뷽���У�
��2�����̬���е�1mg��ת����������еĵ���������______mg������ȷ0.01��
��3��ȡ100mL��ȫ��Ӧ����Һ������19.2gͭ�ۼ�һ������ϡ���ᣬǡ����ȫ��Ӧ�����軹ԭ����ȫ����NO���壩����ԭ��Һ�� ��Ũ��Ϊ______mo1/L�������跴Ӧǰ����Һ��������䣩
��Ũ��Ϊ______mo1/L�������跴Ӧǰ����Һ��������䣩
�⣺��1������Ϣ��֪��O2��NH4+����ΪNO3-�����ݵ���غ��֪��������H+����Ӧ��NH4+��NO3-��NԪ�ػ��ϼ���-3������Ϊ+5�ۣ����ϼ��ܹ�����8�ۣ�O2�� ��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1���������ӷ���ʽΪNH4++2O2=NO3-+2H++H2O��
��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1���������ӷ���ʽΪNH4++2O2=NO3-+2H++H2O��
�ʴ�Ϊ��1NH4+��2O2��1NO3-��2H+��1H2O��
��2������Ҫ����������Ϊx����
NH4++2O2=NO3-+2H++H2O��
18g 64g
1mg�� x
x
����x= ��1mg��
��1mg�� =4.57g
=4.57g
�ʴ�Ϊ��4.57��
��3����Ӧ��NO3-��NO��NԪ�ػ��ϼ���+5�۽���Ϊ+2�ۣ�Cu��Cu2+��CuԪ�ػ��ϼ���0������Ϊ+2�ۣ����ݵ���ת���غ���2n��Cu��=3n��NO3-��= ��2=0.6mol������n����NO3-��=0.2mol������NԪ���غ���n��NH4+��=n��NO3-��=0.2mol������ԭ��Һ��NH4+��Ũ��Ϊ
��2=0.6mol������n����NO3-��=0.2mol������NԪ���غ���n��NH4+��=n��NO3-��=0.2mol������ԭ��Һ��NH4+��Ũ��Ϊ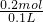 =2mol/L��
=2mol/L��
�ʴ�Ϊ��2��
��������1������Ϣ��֪��O2��NH4+����ΪNO3-�����ݵ���غ��֪��������H+����Ӧ��NH4+��NO3-��NԪ�ػ��ϼ���-3������Ϊ+5�ۣ����ϼ��ܹ�����8�ۣ�O2�� ��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1��
��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1��
��2���̬���е�1mg������NH4+������Ϊ1mg�� �����ݣ�1�������ӷ���ʽ������Ҫ������������
�����ݣ�1�������ӷ���ʽ������Ҫ������������
��3����Ӧ��NO3-��NO��NԪ�ػ��ϼ���+5�۽���Ϊ+2�ۣ�Cu��Cu2+��CuԪ�ػ��ϼ���0������Ϊ+2�ۣ����ݵ���ת���غ���2n��Cu��=3n��NO3-��������NԪ���غ���n��NH4+��=n��NO3-�����ٸ���c= ���㣮
���㣮
����������������ԭ��Ӧ����ƽ�����ݷ���ʽ�ļ��㡢���ʵ���Ũ�ȼ���ȣ��Ѷ��еȣ������غ��ж������������ǽ���Ĺؼ������ջ��ϼ���������ƽ������ԭ��Ӧ����ʽ��
 ��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1���������ӷ���ʽΪNH4++2O2=NO3-+2H++H2O��
��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1���������ӷ���ʽΪNH4++2O2=NO3-+2H++H2O���ʴ�Ϊ��1NH4+��2O2��1NO3-��2H+��1H2O��
��2������Ҫ����������Ϊx����
NH4++2O2=NO3-+2H++H2O��
18g 64g
1mg��
 x
x����x=
 ��1mg��
��1mg�� =4.57g
=4.57g�ʴ�Ϊ��4.57��
��3����Ӧ��NO3-��NO��NԪ�ػ��ϼ���+5�۽���Ϊ+2�ۣ�Cu��Cu2+��CuԪ�ػ��ϼ���0������Ϊ+2�ۣ����ݵ���ת���غ���2n��Cu��=3n��NO3-��=
 ��2=0.6mol������n����NO3-��=0.2mol������NԪ���غ���n��NH4+��=n��NO3-��=0.2mol������ԭ��Һ��NH4+��Ũ��Ϊ
��2=0.6mol������n����NO3-��=0.2mol������NԪ���غ���n��NH4+��=n��NO3-��=0.2mol������ԭ��Һ��NH4+��Ũ��Ϊ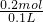 =2mol/L��
=2mol/L���ʴ�Ϊ��2��
��������1������Ϣ��֪��O2��NH4+����ΪNO3-�����ݵ���غ��֪��������H+����Ӧ��NH4+��NO3-��NԪ�ػ��ϼ���-3������Ϊ+5�ۣ����ϼ��ܹ�����8�ۣ�O2��
 ��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1��
��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1����2���̬���е�1mg������NH4+������Ϊ1mg��
 �����ݣ�1�������ӷ���ʽ������Ҫ������������
�����ݣ�1�������ӷ���ʽ������Ҫ��������������3����Ӧ��NO3-��NO��NԪ�ػ��ϼ���+5�۽���Ϊ+2�ۣ�Cu��Cu2+��CuԪ�ػ��ϼ���0������Ϊ+2�ۣ����ݵ���ת���غ���2n��Cu��=3n��NO3-��������NԪ���غ���n��NH4+��=n��NO3-�����ٸ���c=
 ���㣮
���㣮����������������ԭ��Ӧ����ƽ�����ݷ���ʽ�ļ��㡢���ʵ���Ũ�ȼ���ȣ��Ѷ��еȣ������غ��ж������������ǽ���Ĺؼ������ջ��ϼ���������ƽ������ԭ��Ӧ����ʽ��

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ




