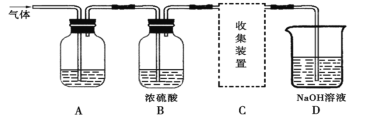
��Ŀ����
����Ŀ��5�ֹ�������A��B��C��D��E���±��в�ͬ������������ɣ����Ǿ�������ˮ��

�ֱ�ȡ���ǵ�ˮ��Һ����ʵ�飬������£�
��C��E��Һ�Լ��ԣ�A��B��D��Һ�����ԣ�0.1mol��L��E��ҺpH��13��
��B��Һ��E��Һ��Ϻ�������ɫ������ͬʱ�����������壻
������C��Һ��D��Һ��Ϻ������ɫ����������C��Һ��D��Һ��Ϻ�������
�ܽ�38.4 g CuƬͶ��װ������D��Һ���Թ��У�Cu���ܽ⣬�ٵμ�1.6 mol��L��1ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֡�
��1���ݴ��ƶ�C��D�Ļ�ѧʽΪ��C______________��D_______________��
��2��д��������з�����Ӧ�����ӷ�Ӧ����ʽ____________________________��
��3�����������Ҫ��CuƬ��ȫ�ܽ⣬���ټ���ϡH2SO4�������____________mL��
��4������ȷ������ҺΪB��______________(����ĸ��ţ���
���𰸡�Ba(OH��2Al(NO3��32Fe3++3CO32-+3H2O=2Fe(OH��3��+3CO2��500A
��������
����������Ϣ��֪��C��E��Һ�Լ��ԣ���Һ����Ϊ����Һ��ǿ�������Σ�A��B��D��Һ�����ԣ�0.1mol��L��E��ҺpH��13����E�����������ˮ�⣬�������ӹ����֪��E����̼������ӣ�������ӹ��棬Eֻ��Ϊ̼���ƣ�������ӹ����֪��CΪ������������B��Һ��E��Һ��Ϻ�������ɫ������ͬʱ�����������壬��B�к��������ӣ���������̼������ӷ���˫ˮ�ⷴӦ���ɶ�����̼�������������ɫ������������C��Һ��D��Һ��Ϻ������ɫ��������������������Һ��D��Һ��Ϻ�������˵��D�к��������ӣ��Ҳ��������������ܽ�38.4 g CuƬͶ��װ������D��Һ���Թ��У�Cu���ܽ⣬�ٵμ�1.6 mol��L��1ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣�˵��D�к�����������ӣ���DΪ����������ôAΪ����ͭ�����Ȼ�ͭ����BΪ�Ȼ�������������
(1)�����Ϸ�����֪��CΪBa(OH��2��DΪAl(NO3��3 �� (2) �����Ϊ̼���ƺ������ӵ�˫ˮ�ⷴӦ�����ӷ���ʽΪ��2Fe3++3CO32-+3H2O=2Fe(OH��3��+3CO2����(3)38.4��ͭ�����ʵ���Ϊ38.4/64=0.6mol��������з��������ӷ�Ӧ����ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O����Ҫ��ͭƬ��ȫ�ܽ⣬��Ҫ�����ӵ����ʵ���Ϊ1.6mol�������ټ���ϡ����������ΪV����1.6��V��2=1.6��V=500mL���ɷ�����֪AΪ����ͭ�����Ȼ�ͭ����BΪ�Ȼ�������������A��B������ȷ����

����Ŀ����ҵ����COΪԭ�����������ѵķ�ӦΪ��3H2(g)��3CO(g) ![]() CH3OCH3(g)��CO2(g) ��H��a kJmol-1 T��ʱ����ʼʱ�ں����ܱ������м���һ������H2��CO��ʵ�����ݺͽ�����±�����ͼ��ʾ��
CH3OCH3(g)��CO2(g) ��H��a kJmol-1 T��ʱ����ʼʱ�ں����ܱ������м���һ������H2��CO��ʵ�����ݺͽ�����±�����ͼ��ʾ��
ʵ�� ��� | ���� ��� | ��ʼ���ʵ��� | ��ƽ��ʱ �ų����� | |
H2 | CO | |||
�� | 2L | 8mol | 8mol | 494 kJ |
�� | 2L | 4mol | 4mol | ���� |

��1��������Ӧƽ�ⳣ��K�ı���ʽΪ_____��
��2���������֪��a��______��b________1(�������������������)��
��3��ʵ����У���Ӧǰ10 min�ڵ�ƽ������v(H2)��_____��
��4������������ʹ������Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����______ (��д�����ĸ)��
a����ʱ�����CH3OCH3����b�������������ݻ����䣬�ٳ���1 mol CO��1 mol H2
c���ʵ������¶� d�������������ݻ����䣬����1 mol ����
��5��T��ʱ���������к�1 molL-1 H2��2 molL-1 CO��2 molL-1 CH3OCH3��3 molL-1 CO2�����ʱv��________v��(�������������������)��