��Ŀ����
����Ŀ��̼��Ԫ�صĵ��ʺͻ�������зdz���Ҫ�����á���ش��������⡣
��1��̼��Ԫ����������������뵼����������__(��Ԫ������)����۵����Ų�ʽΪ___��
��2��CH3OH������Cԭ�ӵ��ӻ���ʽΪ__��SCN-�Ŀռ乹��Ϊ___��
��3��������(CnH2n+2)��n���������۷е����ߣ�ԭ����__��
�ڹ���̼ͬ�壬Ҳ��ϵ���⻯�������������������϶����٣�ԭ����__��
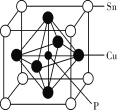
��4����ͼ��SiO2���������ɶ������辧��ṹ����С������__��ԭ�ӹ��ɡ���֪��������Ϊapm�����侧���ܶ�Ϊ__g��cm-3��

���𰸡��� 4s24p2 sp3�ӻ� ֱ���� ����������ɺͽṹ���ƣ�����nֵ������Է������������Ӽ������������۷е����� Si-Si����Si-H�����ܱȽ�С�����ѣ������γɳ��Ĺ��� 12 ![]()
��������
��1��������������뵼�������ĵ�̼��Ԫ�����࣬����32��Ԫ�أ�λ�ڵ������ڣ���۵����Ų�ʽΪ��4s24p2���ʴ�Ϊ���ࣻ4s24p2��
��2��CH3OH�У��۲���Ӷ���=�Ҽ����Ӷ�����4��+����ԭ���ϵŵ��Ӷ�����0��=4������̼ԭ�Ӳ�ȡsp3�ӻ���SCN-������Cԭ�ӣ��۲���Ӷ���=�Ҽ����Ӷ�����2��+����ԭ���ϵŵ��Ӷ�����0��=2������SCN-��C���ӻ���ʽΪsp�ӻ���SCN-�Ŀռ乹��Ϊֱ���ͣ��ʴ�Ϊ��sp3�ӻ���ֱ���ͣ�
��3��������������ɺͽṹ���ƣ�����nֵ������Է������������Ӽ������������ۻ�������ʱ������˷��ķ��Ӽ������������۷е����ߣ��ʴ�Ϊ������������ɺͽṹ���ƣ�����nֵ������Է������������Ӽ������������۷е����ߣ�
�ڱ���C-C����C-H����Si-Si����Si-H�����ܱȽ�С�����ѣ������γɳ��Ĺ���������C-C�ǿ����γɺܳ�̼���ģ��ʴ�Ϊ��Si-Si����Si-H�����ܱȽ�С�����ѣ������γɳ��Ĺ�����
��4����SiO2�������������ɶ������辧��ṹ����С������6��Si��6��O����12��ԭ�ӣ���̯���ɵã�ÿ��SiO2������Si�ĸ���=8��![]() +6
+6![]() +4=8��Oԭ����=16������1mol����������=(28��8+16��16)g=480g����ôÿ������������m=
+4=8��Oԭ����=16������1mol����������=(28��8+16��16)g=480g����ôÿ������������m=![]() ����Ϊ��������=apm=a��10-10cm������ÿ�����������V=(a��10-10cm)3����
����Ϊ��������=apm=a��10-10cm������ÿ�����������V=(a��10-10cm)3����![]() =
=![]() =
=![]() g��cm-3���ʴ�Ϊ��12��
g��cm-3���ʴ�Ϊ��12��![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������п�̸ɵ����һ��һ���Ե�أ����Ϊ����п���м���̼��������Χ����̼�ۡ�MnO2��ZnCl2��NH4Cl����ɵĺ�״�����õ�طŵ���̲���MnOOH�����մ����÷ϵ�ؿɵõ����ֻ���ԭ�ϡ��й��������±���ʾ��
������ | Zn(OH)2 | Fe(OH)2 | Fe(OH)3 |
Ksp����ֵ | 10��17 | 10��17 | 10��39 |
(1)Fe(OH)3�����ܽ�ƽ�ⳣ��(Ksp)�ı���ʽ��ʲô��__________________��
(2)Fe(OH)3�ܷ�����ϡ���________�����ó����ܽ�ƽ�����۽��͡�____________��
(3)�����£���ʹFeCl3��Һ�е�Fe3��������ȫ�������NaOH��Һ������Һ��pHΪ���٣�(����Ũ��С��1��10��5 mol��L��1ʱ��������Ϊ�����ӳ�����ȫ)_____________��
(4)������ʵ���Ũ�ȵ�Zn2����Fe3���Ļ����Һ����μ���NaOH��Һ�����Ȳ����ij�����ʲô��_____________��
(5)����ZnCl2��Һ�л���������Fe2����Ӧ��γ�ȥ��_________��
����Ŀ����ҵ���������ࣨ��Ҫ��S��Se��Fe2O3��CuO��ZnO��SiO2�ȣ�Ϊԭ����ȡ����������ͼ��

��1���������������У��¶ȿ�����95�棬ԭ����___��
��2���������������У�Seת����H2SeO3���÷�Ӧ�Ļ�ѧ����ʽΪ___��
��3������ԭ��������ͨ�����Ƶ�λ��ԭ�ķ�������λ�ߵ������Ȼ�ԭ����λ�͵����ʱ�������Һ�С��±��ǽ������ˢ���������Һ���������ʻ�ԭ��Ӧ�ĵ�λ�����Ƶ�λ��0.782~1.692V���ɳ�ȥ�����ˢ���������Һ�в�����ClO2��
���� | Cu2+/Cu | Zn2+/Zn | Fe2+/Fe | Fe3+/Fe2+ | ClO2/Cl- | H2SeO3/Se |
��λ/V | 0.435 | -0.885 | -0.463 | 0.782 | 1.692 | 0.743 |
Ϊʹ�������ʽ������룬����Na2SO3��ԭʱ����λӦ������___��Χ��H2SeO3�����ᣩ��ԭΪ�������ӷ�Ӧ����ʽΪ___��
��4����Һ������Ҫ���ڵĽ�����������Zn2+��Na+��___��
��5�����ô����辫�ơ����������Һ�м���Na2S��Һ���Խ�������Fe2+������������ת��Ϊ��������ȥ����֪25��ʱKsp(FeS)=6.0��10-18��Ҫʹ��Һ��Fe2+������ȫ[c(Fe2+)��1.0��10-5mol��L-1]�����������Һ��c(S2-)��___mol��L-1��