��Ŀ����
��98%��ŨH2SO4����=1.84g/cm3������500ml 0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��1������ŨH2SO4�����Ϊ______��
��2�����ʵ������10mL��20mL��50mL��Ͳ��Ӧѡ��______ mL��Ͳ��ʵ���л���Ҫ�õ����������ձ�����������______��500mL����ƿ��
��3��������ƿʹ�÷����У����в�������ȷ���ǣ�����ţ�______
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô���Һ��ϴ
C��ȷ��ȡŨ������Һ������ע������ƿ��
D�����ݺ�����ƿ����������ƿ��תҡ��
��4����ʵ���г������������������ҺŨ����ʲôӰ�죿����ƫ�ߡ�ƫ�͡���Ӱ�죩
��Ũ�����ܽ��δ�������¼����ж���______��
���ܽ���Һ���ձ�û��ϴ��______��
��ת��ǰ������ƿ�к�����������ˮ______��
�ܶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶���______��
�ݶ���ʱ�����ӿ̶���______��
��5��������ʱҺ����ڿ̶���Ӧ��ȡ�Ĵ�ʩ��______��
�⣺��1��ŨH2SO4�����ʵ���Ũ��c=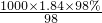 mol/L=18.4mol/L��
mol/L=18.4mol/L��
����Ũ��������ΪV������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬����V��18.4mol/L=500mL��0.5mol/L����ã�V=13.6ml��
�ʴ�Ϊ��13.6ml��
��2����Ũ��������Ϊ13.6ml��������Ҫѡ��20ml��Ͳ��
���Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ���ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ�����������ձ�����ͷ�ιܡ�20mL��Ͳ��500mL����ƿ��
�ʴ�Ϊ��20����ͷ�ιܣ�
��3��A����������跴���ߵ�ҡ�ȣ���ʹ������ƿǰ������Ƿ�©ˮ����A��ȷ��
B������ƿ������ˮϴ�������ô���Һ��ϴ������������ҺŨ��ƫ��B��ȷ��
C��Ũ����ϡ�ͷų��������ȣ���ȴ����������Һ�����ƫС��������ƿ���Ȳ��������ܵ�������Σ�գ�����������ƿ���ܽ��������ƣ���C����
D��ҡ��ʱʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��������ҡ�ȣ���D��ȷ��
��ѡC��
��4����δ����ȴ���Ƚ���Һע������ƿ�У�������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��ϡ���������ձ�δϴ�ӣ��������ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
������ƿ��ԭ����������ˮ�������ʵ����ʵ�������Һ�������Ӱ�죬�������Ƶ���ҺŨ����Ӱ�죬
�ʴ�Ϊ����Ӱ�죻
��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ��������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�ݶ���ʱ���ӿ̶��ߣ���Һ���ƫС������Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��5������ʱҺ����ڿ̶��ߣ�Ӧ������Һ���������ƣ�
�ʴ�Ϊ��������Һ���������ƣ�
��������1������c=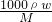 �����Ũ��������ʵ���Ũ�ȣ��ٸ���ϡ�Ͷ��ɼ�������Ũ����������
�����Ũ��������ʵ���Ũ�ȣ��ٸ���ϡ�Ͷ��ɼ�������Ũ����������
��2������Ũ��������ѡ����Ͳ�Ĺ����������Һ��ʵ���������ѡ������������
��3��A����������跴���ߵ�ҡ�ȣ�
B������ƿ������ˮϴ�������ô���Һ��ϴ������������ҺŨ��ƫ��
C��Ũ����ϡ�ͷų��������ȣ���ȴ����������Һ�����ƫС��������ƿ���Ȳ��������ܵ�������Σ�գ�
D��ҡ��ʱʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��������ҡ�ȣ�
��4���������������ʵ����ʵ�������Һ�������Ӱ�죬����c= �ж϶�Ũ�ȵ�Ӱ�죻
�ж϶�Ũ�ȵ�Ӱ�죻
��5������ʱҺ����ڿ̶��ߣ�Ӧ������Һ���������ƣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��Ѷ��еȣ�����c= ������Һ����ԭ������������ע��Ũ�����ϡ�Ͳ�����
������Һ����ԭ������������ע��Ũ�����ϡ�Ͳ�����
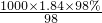 mol/L=18.4mol/L��
mol/L=18.4mol/L������Ũ��������ΪV������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬����V��18.4mol/L=500mL��0.5mol/L����ã�V=13.6ml��
�ʴ�Ϊ��13.6ml��
��2����Ũ��������Ϊ13.6ml��������Ҫѡ��20ml��Ͳ��
���Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ���ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ�����������ձ�����ͷ�ιܡ�20mL��Ͳ��500mL����ƿ��
�ʴ�Ϊ��20����ͷ�ιܣ�
��3��A����������跴���ߵ�ҡ�ȣ���ʹ������ƿǰ������Ƿ�©ˮ����A��ȷ��
B������ƿ������ˮϴ�������ô���Һ��ϴ������������ҺŨ��ƫ��B��ȷ��
C��Ũ����ϡ�ͷų��������ȣ���ȴ����������Һ�����ƫС��������ƿ���Ȳ��������ܵ�������Σ�գ�����������ƿ���ܽ��������ƣ���C����
D��ҡ��ʱʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��������ҡ�ȣ���D��ȷ��
��ѡC��
��4����δ����ȴ���Ƚ���Һע������ƿ�У�������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��ϡ���������ձ�δϴ�ӣ��������ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
������ƿ��ԭ����������ˮ�������ʵ����ʵ�������Һ�������Ӱ�죬�������Ƶ���ҺŨ����Ӱ�죬
�ʴ�Ϊ����Ӱ�죻
��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ��������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�ݶ���ʱ���ӿ̶��ߣ���Һ���ƫС������Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��5������ʱҺ����ڿ̶��ߣ�Ӧ������Һ���������ƣ�
�ʴ�Ϊ��������Һ���������ƣ�
��������1������c=
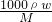 �����Ũ��������ʵ���Ũ�ȣ��ٸ���ϡ�Ͷ��ɼ�������Ũ����������
�����Ũ��������ʵ���Ũ�ȣ��ٸ���ϡ�Ͷ��ɼ�������Ũ������������2������Ũ��������ѡ����Ͳ�Ĺ����������Һ��ʵ���������ѡ������������
��3��A����������跴���ߵ�ҡ�ȣ�
B������ƿ������ˮϴ�������ô���Һ��ϴ������������ҺŨ��ƫ��
C��Ũ����ϡ�ͷų��������ȣ���ȴ����������Һ�����ƫС��������ƿ���Ȳ��������ܵ�������Σ�գ�
D��ҡ��ʱʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��������ҡ�ȣ�
��4���������������ʵ����ʵ�������Һ�������Ӱ�죬����c=
 �ж϶�Ũ�ȵ�Ӱ�죻
�ж϶�Ũ�ȵ�Ӱ�죻��5������ʱҺ����ڿ̶��ߣ�Ӧ������Һ���������ƣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��Ѷ��еȣ�����c=
 ������Һ����ԭ������������ע��Ũ�����ϡ�Ͳ�����
������Һ����ԭ������������ע��Ũ�����ϡ�Ͳ����� 
��ϰ��ϵ�д�
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
 ��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ� ��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�