��Ŀ����
����TiO2��Ϊһ�ֹ����Խ��Խ�ܵ����ǵĹ�ע�������㷺�������ã�
��1����ȡ����TiO2�ķ����ܶ࣬��������ˮ�ⷨ�ǽ�TiCl4���嵼�����������У�700��1000�棩����ˮ�⣬�仯ѧ��ӦʽΪ��______��
��2�����������ѿɹ��ӷ����л���Ⱦ�VOCs��������ˮ�������ڣ�������ϩ���ⷴӦΪ��C2HCl3+2O2��2CO2+HCl+Cl2�������㹻���Ľ�����β����ʵ���Ҽ���������������ļ����ǣ�______��ͨ�������Ƿ��ֻ��ж��ָ��������֮һΪ�� ������л���˴Ź���������______���壮
������л���˴Ź���������______���壮

��3�����ð뵼����TiO2��Ⱦ�ϡ����缫�� ��I-�Ļ����������ʣ�I2+I-?I3-�����ɹ���Ⱦ������̫���ܵ�أ�DSSCs������ԭ����ͼ1���õ�ع���ʱ�������ĵ缫��ӦΪ��______��
��I-�Ļ����������ʣ�I2+I-?I3-�����ɹ���Ⱦ������̫���ܵ�أ�DSSCs������ԭ����ͼ1���õ�ع���ʱ�������ĵ缫��ӦΪ��______��
��4���ڲ�ͬ�����壨��Ƭ����Ƭ���մɣ������Ʊ��������ѱ�Ĥ�������첻ͬ����TiO2��Ĥ���ʹ������ɫ��ÿ�ι���20minȡһ������ʵ������ͼ2������˵����ȷ����______��
��a����ͬ���壬���ۺ����¶�һ������Ƭ���
��b��Լ��520��ʱ����Ƭ����Ĺ���������
��c�����ۺ������壬�������������¶ȵ����߶�����
��d����ͬ����TiO2��Ĥ�Ĺ�����Բ�ͬ��
�⣺��1��TiCl4ˮ�����ɶ������Ѻ��Ȼ��⣬��ѧ��ӦʽΪTiCl4+2H2O TiO2+4HCl���ʴ�Ϊ��TiCl4+2H2O
TiO2+4HCl���ʴ�Ϊ��TiCl4+2H2O TiO2+4HCl[��TiCl4��g��+2H2��g��+O2��g��
TiO2+4HCl[��TiCl4��g��+2H2��g��+O2��g��  TiO2��s��+4HCl��g��]��
TiO2��s��+4HCl��g��]��
��2����������ǿ�����ԣ�����KI��Ӧ���ɵⵥ�ʣ������������ɫ�����Կɽ�ʪ��ĵ���KI��ֽ���ڼ���ƿ�ڣ�֤������������������3���⣬�˴Ź���������3���壬�ʴ�Ϊ����ʪ���KI-������ֽ���飻3��
��3����ͼ���ӵ��ƶ������֪���뵼����TiO2��Ⱦ��Ϊԭ��صĸ��������缫Ϊԭ��ص������������ΪI3-��I-�Ļ���I3-�������ϵõ��ӱ���ԭ��������ӦΪI3-+2e-=3I-���ʴ�Ϊ��I3-+2e-=3I- ��д�ɣ�I2+2e-=2I-���ɲ��۷֣���
��4��A����ͼ֪��500��������Ƭ����A����
B����ͼ֪����520��ʱ����ɫ����ߣ���Ƭ����Ĺ��������ã���B��ȷ��
C��520�����Ƭ���������¶ȵ����߶��ϵͣ���C����
D����ͼ֪����ͬ�¶�ʱ����ͬ�����壨��Ƭ����Ƭ���մɣ���TiO2��Ĥ�Ĺ�����Բ�ͬ����D��ȷ��
��ѡbd��
��������1��TiCl4ˮ�����ɶ������Ѻ��Ȼ��⣻
��2����������ǿ�����ԣ�����KI��Ӧ���ɵⵥ�ʣ������������ɫ���������м����⣬�˴Ź��������м��ַ壻
��3����ͼ���ӵ��ƶ������֪���뵼����TiO2��Ⱦ��Ϊԭ��صĸ��������缫Ϊԭ��ص������������ΪI3-��I-�Ļ���I3-�������ϵõ��ӱ���ԭ��
��4������ͼ����ɫ�����¶ȹ�ϵ���⣻
���������⿼��ԭ��صĹ���ԭ������Ŀ�ѶȲ�����ע����յ缫��Ӧʽ����д��ͼ�е��ӵ��ƶ������ǽ��Ĺؼ���
 TiO2+4HCl���ʴ�Ϊ��TiCl4+2H2O
TiO2+4HCl���ʴ�Ϊ��TiCl4+2H2O TiO2+4HCl[��TiCl4��g��+2H2��g��+O2��g��
TiO2+4HCl[��TiCl4��g��+2H2��g��+O2��g��  TiO2��s��+4HCl��g��]��
TiO2��s��+4HCl��g��]����2����������ǿ�����ԣ�����KI��Ӧ���ɵⵥ�ʣ������������ɫ�����Կɽ�ʪ��ĵ���KI��ֽ���ڼ���ƿ�ڣ�֤������������������3���⣬�˴Ź���������3���壬�ʴ�Ϊ����ʪ���KI-������ֽ���飻3��
��3����ͼ���ӵ��ƶ������֪���뵼����TiO2��Ⱦ��Ϊԭ��صĸ��������缫Ϊԭ��ص������������ΪI3-��I-�Ļ���I3-�������ϵõ��ӱ���ԭ��������ӦΪI3-+2e-=3I-���ʴ�Ϊ��I3-+2e-=3I- ��д�ɣ�I2+2e-=2I-���ɲ��۷֣���
��4��A����ͼ֪��500��������Ƭ����A����
B����ͼ֪����520��ʱ����ɫ����ߣ���Ƭ����Ĺ��������ã���B��ȷ��
C��520�����Ƭ���������¶ȵ����߶��ϵͣ���C����
D����ͼ֪����ͬ�¶�ʱ����ͬ�����壨��Ƭ����Ƭ���մɣ���TiO2��Ĥ�Ĺ�����Բ�ͬ����D��ȷ��
��ѡbd��
��������1��TiCl4ˮ�����ɶ������Ѻ��Ȼ��⣻
��2����������ǿ�����ԣ�����KI��Ӧ���ɵⵥ�ʣ������������ɫ���������м����⣬�˴Ź��������м��ַ壻
��3����ͼ���ӵ��ƶ������֪���뵼����TiO2��Ⱦ��Ϊԭ��صĸ��������缫Ϊԭ��ص������������ΪI3-��I-�Ļ���I3-�������ϵõ��ӱ���ԭ��
��4������ͼ����ɫ�����¶ȹ�ϵ���⣻
���������⿼��ԭ��صĹ���ԭ������Ŀ�ѶȲ�����ע����յ缫��Ӧʽ����д��ͼ�е��ӵ��ƶ������ǽ��Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
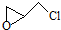 ������л���˴Ź���������
������л���˴Ź���������

 w ww.k s5u. co m
w ww.k s5u. co m
 C6H12O6�������ǣ�+6O2��
C6H12O6�������ǣ�+6O2��

 C6H12O6�������ǣ�+6O2��
C6H12O6�������ǣ�+6O2��