��Ŀ����
��һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl����C1O����C1O3�����ֺ���Ԫ�ص����ӣ�����C1O����C1O3���������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��

��1��t2ʱ��Ca(OH)2��Cl2������Ӧ���ܻ�ѧ����ʽΪ��____________ ��
��2����ʯ�����к���Ca(OH)2�����ʵ�����______ mol��
��3���ݷ���������Ca(C1O3)2�ķ�Ӧ�����¶���������ģ�ͨ��Cl2���ٶȲ�ͬ��C1O����C1O3���ı���Ҳ��ͬ��
����ԭʯ������ͨ���������ٶȼӿ죬��Ӧ��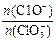 2���>������<����=������
2���>������<����=������
�� ����n(C1��)= mol���ú�a�Ĵ���ʽ����ʾ����
����n(C1��)= mol���ú�a�Ĵ���ʽ����ʾ����

��1��t2ʱ��Ca(OH)2��Cl2������Ӧ���ܻ�ѧ����ʽΪ��____________ ��
��2����ʯ�����к���Ca(OH)2�����ʵ�����______ mol��
��3���ݷ���������Ca(C1O3)2�ķ�Ӧ�����¶���������ģ�ͨ��Cl2���ٶȲ�ͬ��C1O����C1O3���ı���Ҳ��ͬ��
����ԭʯ������ͨ���������ٶȼӿ죬��Ӧ��
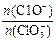 2���>������<����=������
2���>������<����=��������
 ����n(C1��)= mol���ú�a�Ĵ���ʽ����ʾ����
����n(C1��)= mol���ú�a�Ĵ���ʽ����ʾ������12�֣��� 10Ca(OH)2��10Cl2��2Ca(C1O)2��Ca(C1O3)2��7CaCl2��10H2O ��4�֣�
�� 5 ��2�֣� �� ������2�֣� ��4�֣�
��4�֣�
�� 5 ��2�֣� �� ������2�֣�
 ��4�֣�
��4�֣���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ɱ���ࡣ�䴦����������������ʹ��ת��Ϊ�Ͷ�������أ�KCNO�������������ô������Σ������ɵ�CNO-�ɽ�һ������Ϊ��ֱ���ŷŵ������е������塣�����о�����ijЩ�����С����������Һ�У��ڹ�������£���С������Һ���淢��������ԭ��Ӧ����С�������������ʲ������仯����������ѣ�TiO2��С������Ϳ����ƻ��軯����ж����
ɱ���ࡣ�䴦����������������ʹ��ת��Ϊ�Ͷ�������أ�KCNO�������������ô������Σ������ɵ�CNO-�ɽ�һ������Ϊ��ֱ���ŷŵ������е������塣�����о�����ijЩ�����С����������Һ�У��ڹ�������£���С������Һ���淢��������ԭ��Ӧ����С�������������ʲ������仯����������ѣ�TiO2��С������Ϳ����ƻ��軯����ж����
