��Ŀ����
����Ŀ���л���A�ǾۺϷ�Ӧ�����������ϵĵ��壬�����Ϊ�ϳɵ����I����������J��ԭ�ϣ���غϳ�·�����£�

��֪��������ͼ����A������ʺɱ�Ϊ118���䱽���ϵ�һ�ȴ��ﹲ���֣��˴Ź���������
ʾ�������Ϊ3:2:2:2:1��
����������Ϣ�ش��������⣺
��1��A�Ĺ���������Ϊ__________________��B��C�ķ�Ӧ����Ϊ_____________��E��F�ķ�Ӧ����Ϊ_____________��
��2��I�Ľṹ��ʽΪ____________________����K�����к���������Ԫ��״�ṹ���������ʽΪ________________��
��3��D������������ͭ����Һ��Ӧ�����ӷ���ʽΪ_______________________________��
��4��H��ͬ���칹��W����Ũ��ˮ��Ӧ������ɫ������1 mol W���뷴Ӧ�������3 mol Br2����д�����з���������W�Ľṹ��ʽ___________________________________��
��5��J��һ�ָ߷��ӻ��������C����J�Ļ�ѧ����ʽΪ
______________________________________________________________________��
��6��![]() ��֪��
��֪��![]() ��RΪ������
��R������
����Ա�����ϩΪ��ʼԭ���Ʊ�H�ĺϳ�·�ߣ����Լ���ѡ����
[�ϳ�·��ʾ����]![]()
_____________________________________________________________________��
���𰸡� ̼̼˫�� ��������ˮ��Һ������ ��ȥ��Ӧ  C18H16O4
C18H16O4 
 ��
��

�������д�ɣ� ��
�� 
����������A����Br2�����ӳɷ�Ӧ��˵�����ӽṹ�к���̼̼˫����������ͼ����A������ʺɱ�Ϊ118��˵����Է�������Ϊ118������ʽΪC9H10���䱽���ϵ�һ�ȴ��ﹲ���֣����ܱ�����ֻ��һ��ȡ�������˴Ź���������ʾ�������Ϊ3:2:2:2:1����֪A�Ľṹ��ʽΪ![]() ��A��Br2�����ӳɷ�Ӧ����BΪ
��A��Br2�����ӳɷ�Ӧ����BΪ ��B����������ˮ��Һ�����ȷ���ˮ�����ɵ�CΪ
��B����������ˮ��Һ�����ȷ���ˮ�����ɵ�CΪ ��C���������ɵ�DΪ
��C���������ɵ�DΪ ��D������������ͭ����������Ӧ���ɵ�EΪ
��D������������ͭ����������Ӧ���ɵ�EΪ ��E������ȥ��Ӧ���ɵ�FΪ
��E������ȥ��Ӧ���ɵ�FΪ ��F��
��F��![]() ������Ӧ����IΪ
������Ӧ����IΪ![]() ��
��
��1��![]() �Ĺ���������Ϊ̼̼˫������B��C�����ķ�ӦΪ±������ˮ�⣬�䷴Ӧ����Ϊ��������ˮ��Һ�����ȣ���E��F���������H2O����Ϸ�Ӧ����������Ӧ������ȥ��Ӧ��
�Ĺ���������Ϊ̼̼˫������B��C�����ķ�ӦΪ±������ˮ�⣬�䷴Ӧ����Ϊ��������ˮ��Һ�����ȣ���E��F���������H2O����Ϸ�Ӧ����������Ӧ������ȥ��Ӧ��
��2��I�Ľṹ��ʽΪ![]() ����K�����к���������Ԫ��״�ṹ��˵�������ӵ�
����K�����к���������Ԫ��״�ṹ��˵�������ӵ� �������Ӽ��������ɻ�����2���ӵ�ˮ������ԭ���غ��֪K�ķ���ʽΪC18H16O4��
�������Ӽ��������ɻ�����2���ӵ�ˮ������ԭ���غ��֪K�ķ���ʽΪC18H16O4��
��3�� ������������ͭ����Һ��Ӧ�����ӷ���ʽΪ
������������ͭ����Һ��Ӧ�����ӷ���ʽΪ ��
��
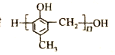
��4��![]() ��ͬ���칹��W����Ũ��ˮ��Ӧ������ɫ������˵�����ӽṹ�к��з��ǻ���1 mol W���뷴Ӧ�������3 molBr2��˵�������Ͽ�ȡ����λ�����ڡ���λ�������з���������W�Ľṹ��ʽ��
��ͬ���칹��W����Ũ��ˮ��Ӧ������ɫ������˵�����ӽṹ�к��з��ǻ���1 mol W���뷴Ӧ�������3 molBr2��˵�������Ͽ�ȡ����λ�����ڡ���λ�������з���������W�Ľṹ��ʽ�� ��
�� ��
��
��5���� ��HOOCCOOH�������۷�Ӧ���ɸ߷��ӻ�����J�Ļ�ѧ����ʽΪ
��HOOCCOOH�������۷�Ӧ���ɸ߷��ӻ�����J�Ļ�ѧ����ʽΪ ��
��
��6���Ա�����ϩΪ��ʼԭ���Ʊ�![]() �ĺϳ�·��Ϊ
�ĺϳ�·��Ϊ ��
��

����Ŀ�������ǽ��������У���һֱ��������Ҫ�Ľ�ɫ��������������Ҫ�ɷ���Si����(����)
A. ˮ��B. ���C. ʯӢD. �����оƬ
����Ŀ��̼���̣�MnCO3��������������ĵ����������壬Ҳ��������Ĵ��������ԡ�Ϳ�Ϻ���������ϡ���ҵ���������̿���Ҫ�ɷ���MnO2��������Fe2O3��CaCO3��CuO�����ʣ���ȡ̼���̵���������ͼ��ʾ��

��֪����ԭ��������ӦΪ��2MnO2+C![]() 2MnO+CO2����
2MnO+CO2����
�����õ����������£�
�������� | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Mn(OH)2 |
��ʼ����pH | 1.5 | 6.5 | 4.2 | 8.3 |
������ȫpH | 3.7 | 9.7 | 7.4 | 9.8 |
����Ҫ��ش��������⣺
��1����ʵ���ҽ��в���A����Ҫ�õ�������Ϊ________________������B����������������Ϊ35%�����ᣨ�ܶ���=1.26 g/cm3�����������ʵ���Ũ��Ϊ_________��
��2������C�еõ���������Ҫ�ɷ���_______________������D�л�ԭ���������������ʵ���֮��Ϊ__________��
��3������E�е���pH�ķ�ΧΪ________����Ŀ����_____________________________��
��4������G�����ӷ���ʽΪ___________________����Mn2+������ȫʱ�����Һ��CO32����Ũ��Ϊ2.2��10��6 mol/L����Ksp(MnCO3)=__________��
��5��ʵ���ҿ�����Na2S2O8��Һ������Mn2+�Ƿ���ȫ������Ӧ��ԭ��Ϊ��
Mn2++S2O82��+H2O=H++SO42��+MnO4����
������ƽ�������ӷ���ʽ____________________________��
��ȷ��Mn2+�����Ѿ���ȫ��Ӧ��������_______________________________��