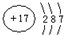��Ŀ����
 ��2013?���죩�Ͻ��ǽ�������ĸ����������ϣ�
��2013?���죩�Ͻ��ǽ�������ĸ����������ϣ���1����ĸ�������������Ͻ����죮
����Ԫ�������ڱ��е�λ����
�������ڵڢ�A��
�������ڵڢ�A��
����ҵ������ԭ������������ȡ���ã���ȡ������ͨ�������ΪCO2
CO2
����Al-Mg�Ͻ�ǰ��NaOH��Һ����Al2O3Ĥ���仯ѧ����ʽΪ
Al2O3+2NaOH=2NaAlO2+H2O
Al2O3+2NaOH=2NaAlO2+H2O
�����ӹ�����ʹ�õı�����ΪAr
Ar
���ѧʽ������2����ĸ�������Ϊ�Ͻ�֣�
�ٽ����ں�ˮ�з����ĵ绯ѧ��ʴ��ҪΪ
������ʴ
������ʴ
���ں�ĸ�øֿ��ɵ�����ұ�����ɣ���������������Ϊ���躬������������Ϊ
CaCO3��CaO
CaCO3��CaO
����3����ĸ��������Ҫ��ͭ�Ͻ����죮
��80.0g Cu-Al�Ͻ�������ȫ�ܽ���������ˮ�����˵õ���ɫ����39.0����Ͻ���Cu����������Ϊ
83.1%
83.1%
����Ϊ����ijͭ�Ͻ�ijɷ֣����Ὣ����ȫ�ܽ����NaOH��Һ����pH����pH=3.4ʱ��ʼ���ֳ������ֱ���pHΪ7.0��8.0ʱ���˳������������ͼ��Ϣ�ƶϸúϽ��г�ͭ��һ������
Al��Ni
Al��Ni
����������1��������13��Ԫ�أ���ԭ����3�����Ӳ㣬����������Ϊ3���ݴ�ȷ�������ڱ��е�λ�ã�
������������NaOH��Һ������ת��Ϊƫ�����Σ���ȥ���ʣ���ͨ�������̼�����������������������������ɵ���������
�����������������Ʒ�Ӧ����ƫ��������ˮ���ں��ӹ�����Ϊ��ֹAl��Mg�Ƚ�������������Ӧ������Ar�ȶ������屣���º��ӣ�
��2���ٽ������Ҫ�ɷ�Ϊ������������̼����̼�Ͻ𣩣���ˮ����Ϊ������Һ������Ҫ����������ʴ��
�������������п��Լ���CaCO3��CaO�����γɹ����Σ���Ϊ¯����ȥ�Ӷ����躬����
��3����ͭ���Ͻ�������ȫ�ܽ���������ˮ��ͭ����ת��Ϊ�İ���ͭ�����Ӽ�����Һ�����˵õ���ɫ��������������������n=
�����������������ʵ������ٸ���m=nM����Al����������������Ͻ���Cu���������ٸ�����������������㣻
��pH=3.4ʱ��ʼ���ֳ���ΪAl��OH��3��pH=8.0ʱ���˳�ȥNi��OH��2����˸�ͭ�Ͻ��л���Al��Ni��
������������NaOH��Һ������ת��Ϊƫ�����Σ���ȥ���ʣ���ͨ�������̼�����������������������������ɵ���������
�����������������Ʒ�Ӧ����ƫ��������ˮ���ں��ӹ�����Ϊ��ֹAl��Mg�Ƚ�������������Ӧ������Ar�ȶ������屣���º��ӣ�
��2���ٽ������Ҫ�ɷ�Ϊ������������̼����̼�Ͻ𣩣���ˮ����Ϊ������Һ������Ҫ����������ʴ��
�������������п��Լ���CaCO3��CaO�����γɹ����Σ���Ϊ¯����ȥ�Ӷ����躬����
��3����ͭ���Ͻ�������ȫ�ܽ���������ˮ��ͭ����ת��Ϊ�İ���ͭ�����Ӽ�����Һ�����˵õ���ɫ��������������������n=
| m |
| M |
��pH=3.4ʱ��ʼ���ֳ���ΪAl��OH��3��pH=8.0ʱ���˳�ȥNi��OH��2����˸�ͭ�Ͻ��л���Al��Ni��
����⣺��1����Al�ĺ�����Ӳ���Ϊ3������������Ϊ3�����λ�����ڱ��е������ڵڢ�A�壻
��ҵұ������ԭ����Al2O3��������������ȡAl2O3�ķ���һ���ǽ�����������NaOH��Һ��ʹAlת��ΪAlO2-��Ȼ��ͨ��CO2����ʹAlO2-ת��ΪAl��OH��3������Ȼ���ٽ�Al��OH��3�������ȼ��ɵõ�Al2O3��
�ʴ�Ϊ���������ڵڢ�A�壻CO2��
�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ����ʽΪ��Al2O3+2NaOH=2NaAlO2+H2O���ں��ӹ�����Ϊ��ֹAl��Mg�Ƚ�������������Ӧ������Ar�ȶ������屣���º��ӣ��ʴ�Ϊ��Al2O3+2NaOH=2NaAlO2+H2O��
��2���ٽ������Ҫ�ɷ�Ϊ������������̼����̼�Ͻ𣩣�����ں�ˮ�����γ�ԭ��ط���������ʴ���ʴ�Ϊ��������ʴ��
��ұ�����̿ɼ���CaCO3��CaO����γɹ����Σ���Ϊ¯����ȥ�Ӷ����躬�����ʴ�Ϊ��CaCO3��CaO��
��3����Cu2+�ڹ�����ˮ�����γ������ӣ���˵õ�39.0g��ɫ����ΪAl��OH��3����������ԭ���غ��֪80.0gCu-Al�Ͻ��к���m��Al��=27g/mol��
=13.5g���ʸúϽ���ͭ����������Ϊ
=83.1%���ʴ�Ϊ��83.1%��
����ͼ��֪�����������Ϣ��֪��ʼ���ֳ���ΪAl��OH��3��pH=8.0ʱ���˳�ȥNi��OH��2����˸�ͭ�Ͻ��л���Al��Ni���ʴ�Ϊ��Al��Ni��
��ҵұ������ԭ����Al2O3��������������ȡAl2O3�ķ���һ���ǽ�����������NaOH��Һ��ʹAlת��ΪAlO2-��Ȼ��ͨ��CO2����ʹAlO2-ת��ΪAl��OH��3������Ȼ���ٽ�Al��OH��3�������ȼ��ɵõ�Al2O3��
�ʴ�Ϊ���������ڵڢ�A�壻CO2��
�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ����ʽΪ��Al2O3+2NaOH=2NaAlO2+H2O���ں��ӹ�����Ϊ��ֹAl��Mg�Ƚ�������������Ӧ������Ar�ȶ������屣���º��ӣ��ʴ�Ϊ��Al2O3+2NaOH=2NaAlO2+H2O��
��2���ٽ������Ҫ�ɷ�Ϊ������������̼����̼�Ͻ𣩣�����ں�ˮ�����γ�ԭ��ط���������ʴ���ʴ�Ϊ��������ʴ��
��ұ�����̿ɼ���CaCO3��CaO����γɹ����Σ���Ϊ¯����ȥ�Ӷ����躬�����ʴ�Ϊ��CaCO3��CaO��
��3����Cu2+�ڹ�����ˮ�����γ������ӣ���˵õ�39.0g��ɫ����ΪAl��OH��3����������ԭ���غ��֪80.0gCu-Al�Ͻ��к���m��Al��=27g/mol��
| 39g |
| 78g/mol |
| 80g-13.5g |
| 80g |
����ͼ��֪�����������Ϣ��֪��ʼ���ֳ���ΪAl��OH��3��pH=8.0ʱ���˳�ȥNi��OH��2����˸�ͭ�Ͻ��л���Al��Ni���ʴ�Ϊ��Al��Ni��
���������⿼�������ʼ��仯�������ʡ�������ʴ������ұ�������ӳ����ܶȻ�����ѧ����ȣ��Ѷ��еȣ��ۺ��Խϴ���Ҫѧ��������ʵ�Ļ�����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ