��Ŀ����
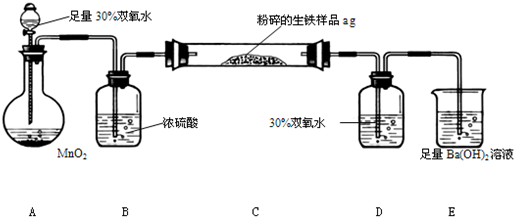
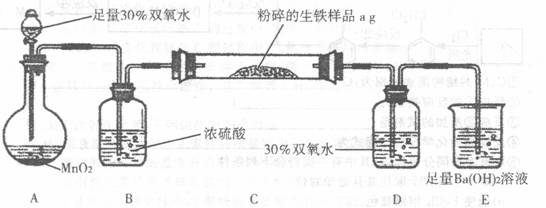
�����г����⣬����������Ԫ�أ���̼Ԫ�غ���Ԫ�أ�����̼��Ҫ��̼��������̬���ڣ�ij��ȤС����ư���ͼ��ʾ��ʵ��װ�ã��ⶨ�����еĺ�̼����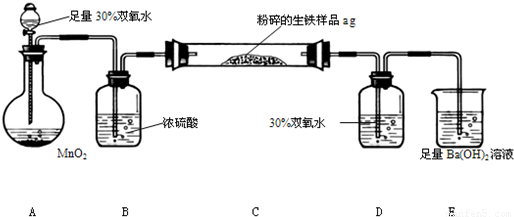
��ش��������⣺
��1���������������к�Ԫ�أ���ʹ���������ȴ��ԣ���Ԫ�������������п��ܴ��ڵļ�̬��
A��-2 B��0 C��+4 D��+6
��2��д�����ձ�E�з�����Ӧ�����ӷ���ʽ�� ��
��3��D��30% ˫��ˮ�������� ������װ�ã����ⶨ�ĺ�̼���� ���ƫ�ߡ�����ƫ�͡���Ӱ�족��
��4����Ӧ��ɺ�����֤����������Ԫ�أ�������Ƶ�ʵ�鷽���ǣ�д��ʵ�鲽�衢���� ��
��5����C�ܵ���Ʒ��ַ�Ӧ���E�����ɵij���Ϊbg�������������еĺ�̼��Ϊ ��
��6��ʵ������У�����ȤС��Ӧע��İ�ȫ������ ������дһ�֣�
���𰸡���������1�����Ļ��ϼ�һ��Ϊ���ۣ���̼�ķǽ���С����ķǽ����ԣ����Sһ����ʾ���ۣ�
��2��װ��A�й��������ڶ�������Ϊ����������������������ͨ��Ũ��������װ��C�е�̼Ԫ������Ϊ������̼����Ԫ������Ϊ����������������ͨ������������Һ�����������������գ�������̼ͨ��Eװ������̼�ᱵ������
��3�����û��˫��ˮ����������������Ҳ��������������Ӧ���ɳ�����ʹ̼�İٷֺ���ƫ�ߣ�
��4������װ��D����Һ���Ƿ��������������֤�����Ƿ�����Ԫ�أ������ǣ�ȡ����Dƿ��Һ���Թ��У��μ������ữ��BaCl2��Һ�������ְ�ɫ������˵�������к�����Ԫ�أ�
��5����E�г���������Ϊbg����̼�ᱵ������������̼Ԫ���غ��֪̼�ᱵ�����ʵ����������̼�����ʵ�����ȣ��ݴ˼���������̼������������������̼������Ϊ��������������������̼����������Ϊ��
��6��ʵ�������Ӧע��İ�ȫ�����У�Ũ�����и�ʴ�ԣ�ʹ��ʱҪС�ģ�����ʱҪʹ��ͨ�ܾ������ȵȣ�
����⣺��1�����Ļ��ϼ�һ��Ϊ���ۣ���̼�ķǽ���С����ķǽ����ԣ����Sһ����ʾ���ۣ�
��ѡ��A��
��2��������̼������������Ӧ����̼�ᱵ��ˮ����Ӧ���ӷ���ʽΪCO2+Ba2++2OH-=BaCO3��+H2O��
�ʴ�Ϊ��CO2+Ba2++2OH-=BaCO3��+H2O��
��3�����û��˫��ˮ����������������Ҳ��������������Ӧ���ɳ�����ʹ̼�İٷֺ���ƫ�ߣ���D��30% ˫��ˮ������������SO2���壮
�ʴ�Ϊ������SO2���壻
��4��ȡ����Dƿ��Һ���Թ��У��μ������ữ��BaCl2��Һ�������ְ�ɫ������˵�������к�����Ԫ�أ�
�ʴ�Ϊ��ȡ����Dƿ��Һ���Թ��У��μ������ữ��BaCl2��Һ�������ְ�ɫ������˵�������к�����Ԫ�أ�
��5����E�еõ�bg̼�ᱵ������̼Ԫ���غ��֪̼�ᱵ�����ʵ����������̼�����ʵ�����ȣ���������̼������Ϊ ×12g/mol=
×12g/mol= g��������̼����������Ϊ
g��������̼����������Ϊ ×100%=
×100%= %��
%��
�ʴ�Ϊ�� %��
%��
��6��ʵ�������Ӧע��İ�ȫ�����У�Ũ�����и�ʴ�ԣ�ʹ��ʱҪС�ģ�����ʱҪʹ��ͨ�ܾ������ȵȣ�
�ʴ�Ϊ��Ũ�����и�ʴ�ԣ�ʹ��ʱҪС�ģ�����ʱҪʹ��ͨ�ܾ������ȵȣ�
�����������ʵ�鷽�������װ�õ����⡢ʵ�������������ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ������֪ʶ�������⡢����������������������Ͷ�����̼������������������������ƣ���˸߿��ж�������������ļ���ıȽ϶࣬�������������Ӧ������һ���㣬��������������ʼ��Կ��飮
��2��װ��A�й��������ڶ�������Ϊ����������������������ͨ��Ũ��������װ��C�е�̼Ԫ������Ϊ������̼����Ԫ������Ϊ����������������ͨ������������Һ�����������������գ�������̼ͨ��Eװ������̼�ᱵ������
��3�����û��˫��ˮ����������������Ҳ��������������Ӧ���ɳ�����ʹ̼�İٷֺ���ƫ�ߣ�
��4������װ��D����Һ���Ƿ��������������֤�����Ƿ�����Ԫ�أ������ǣ�ȡ����Dƿ��Һ���Թ��У��μ������ữ��BaCl2��Һ�������ְ�ɫ������˵�������к�����Ԫ�أ�
��5����E�г���������Ϊbg����̼�ᱵ������������̼Ԫ���غ��֪̼�ᱵ�����ʵ����������̼�����ʵ�����ȣ��ݴ˼���������̼������������������̼������Ϊ��������������������̼����������Ϊ��
��6��ʵ�������Ӧע��İ�ȫ�����У�Ũ�����и�ʴ�ԣ�ʹ��ʱҪС�ģ�����ʱҪʹ��ͨ�ܾ������ȵȣ�
����⣺��1�����Ļ��ϼ�һ��Ϊ���ۣ���̼�ķǽ���С����ķǽ����ԣ����Sһ����ʾ���ۣ�
��ѡ��A��
��2��������̼������������Ӧ����̼�ᱵ��ˮ����Ӧ���ӷ���ʽΪCO2+Ba2++2OH-=BaCO3��+H2O��
�ʴ�Ϊ��CO2+Ba2++2OH-=BaCO3��+H2O��
��3�����û��˫��ˮ����������������Ҳ��������������Ӧ���ɳ�����ʹ̼�İٷֺ���ƫ�ߣ���D��30% ˫��ˮ������������SO2���壮
�ʴ�Ϊ������SO2���壻
��4��ȡ����Dƿ��Һ���Թ��У��μ������ữ��BaCl2��Һ�������ְ�ɫ������˵�������к�����Ԫ�أ�
�ʴ�Ϊ��ȡ����Dƿ��Һ���Թ��У��μ������ữ��BaCl2��Һ�������ְ�ɫ������˵�������к�����Ԫ�أ�
��5����E�еõ�bg̼�ᱵ������̼Ԫ���غ��֪̼�ᱵ�����ʵ����������̼�����ʵ�����ȣ���������̼������Ϊ
 ×12g/mol=
×12g/mol= g��������̼����������Ϊ
g��������̼����������Ϊ ×100%=
×100%= %��
%���ʴ�Ϊ��
 %��
%����6��ʵ�������Ӧע��İ�ȫ�����У�Ũ�����и�ʴ�ԣ�ʹ��ʱҪС�ģ�����ʱҪʹ��ͨ�ܾ������ȵȣ�
�ʴ�Ϊ��Ũ�����и�ʴ�ԣ�ʹ��ʱҪС�ģ�����ʱҪʹ��ͨ�ܾ������ȵȣ�
�����������ʵ�鷽�������װ�õ����⡢ʵ�������������ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ������֪ʶ�������⡢����������������������Ͷ�����̼������������������������ƣ���˸߿��ж�������������ļ���ıȽ϶࣬�������������Ӧ������һ���㣬��������������ʼ��Կ��飮

��ϰ��ϵ�д�
�����Ŀ