��Ŀ����
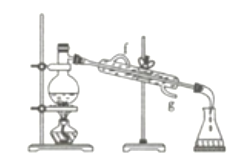
����Ŀ�����������������Ķ��ˮ��������ijʵ��С�������ͼװ���Ʊ��������(K2FeO4)��̽����������;��

��.�Ʊ�K2FeO4(�г֡����ȵ�װ����)

(1)B�������Լ�Ϊ______________________��
(2)C�з�ӦΪ���ȷ�Ӧ������Ӧ�¶��������0~8�棬��ʹ�õĿ��·���Ϊ___________����ַ�Ӧ��õ���ɫ���壬��Ӧ����ʽΪ___________����Ӧ��KOH���������ԭ����___________��
(3)C�л���ᆳ���ˡ�ϴ�ӡ�����ô����{����ؾ��壬ϴ��ʱϴ�Ӽ���ѡ��___________��
a.��ˮ b.KOH��Һ c.�����
��.̽��K2FeO4������
(4)K2FeO4���Խ���ˮ�е�CN������ΪCNO����ʵ�������pH=9ʱCN��ȥ��Ч����ѡ���ƽ��Ӧ���ӷ���ʽ��____FeO42��+____CN��+____H2O��____Fe(OH)3��+____CNO��+___OH����
�ִ�����CN������Ũ��Ϊ13mg/L�ķ�ˮ1m3��������ҪK2FeO4___________g��
(5)���ϱ�����������Һ��������FeO42��>MnO4������֤ʵ�飺����K2FeO4�ܽ��ڹ���KOH��Һ�У���Һ��dz��ɫ��ȡ����Һ����MnSO4��H2SO4�Ļ����Һ�У�����Һ��ɫ��Ȼ��dz��ɫ�������ʵ��֤���������dz��ɫ��Һ�к���MnO4��___________��
���𰸡�����ʳ��ˮ ��ˮԡ����ˮԡ 3Cl2+2Fe(OH)3+10KOH![]() 2K2FeO4+6KCl+8H2O ������Һ���ԣ���ֹK2FeO4���� c 2 3 5 2 3 4 66 ������dz��ɫ��Һ�еμӹ���ϡH2SO4������Һdz��ɫδ��ȥ��
2K2FeO4+6KCl+8H2O ������Һ���ԣ���ֹK2FeO4���� c 2 3 5 2 3 4 66 ������dz��ɫ��Һ�еμӹ���ϡH2SO4������Һdz��ɫδ��ȥ��
��������
��С����ʵ��̽���⡣
��1������������лӷ��ԣ�����Cl2�л���HCl��H2O(g)��HCl������Fe(OH)3��KOH��Ӧ�ñ���ʳ��ˮ��ȥHCl��B�������Լ�Ϊ����ʳ��ˮ��
��2��C�з�ӦΪ���ȷ�Ӧ������Ӧ�¶��������0~8�棬��ʹ�õĿ��·���Ϊ��ˮԡ����ˮԡ����ַ�Ӧ��õ���ɫ����ΪK2FeO4����Ӧ����ʽΪ3Cl2+2Fe(OH)3+10KOH=2K2FeO4+6KCl+8H2O����ΪK2FeO4����KOH���������л��ܼ�������ǿ�����������Ի�������Һ����ɫ������ȥ�������������ڼ�����Һ�н��ȶ������Է�Ӧ��KOH�����������3����ΪK2FeO4����KOH���������л��ܼ���C�л���ᆳ���ˡ�ϴ�ӡ�����õ�����������ؾ��壬ϴ��ʱϴ�Ӽ���ѡ�������
��4��K2FeO4���Խ���ˮ�е�CN������ΪCNO������Ӧ������FeO42������Ԫ�ؼ�̬��+6�۽��͵�+3������Fe(OH)3��CN����̼Ԫ�ؼ�̬��+2�����ߵ�+4������CNO�������ݵ�ʧ�����غ㡢ԭ���غ㡢����غ���ƽ���ӷ���ʽ�����ӷ���ʽΪ��2FeO42��+3CN��+5H2O=2Fe(OH)3��+3CNO��+4OH����
��5����������K2FeO4������H2SO4��Һ�л�ת��ΪFe3+��O2�������Һ�в�����K2FeO4����Һ���dz��ɫһ����MnO4������ɫ��
��1������������лӷ��ԣ�����Cl2�л���HCl��H2O(g)��HCl������Fe(OH)3��KOH��Ӧ�ñ���ʳ��ˮ��ȥHCl��B�������Լ�Ϊ����ʳ��ˮ����С���Ϊ������ʳ��ˮ��
��2��C�з�ӦΪ���ȷ�Ӧ������Ӧ�¶��������0~8�棬��ʹ�õĿ��·���Ϊ��ˮԡ����ˮԡ����ַ�Ӧ��õ���ɫ����ΪK2FeO4����Ӧ����ʽΪ3Cl2+2Fe(OH)3+10KOH=2K2FeO4+6KCl+8H2O����ΪK2FeO4����KOH���������л��ܼ�������ǿ�����������Ի�������Һ����ɫ������ȥ�������������ڼ�����Һ�н��ȶ������Է�Ӧ��KOH�����������С���Ϊ����ˮԡ����ˮԡ��3Cl2+2Fe(OH)3+10KOH=2K2FeO4+6KCl+8H2O��������Һ���ԣ���ֹK2FeO4���ʡ�
��3����ΪK2FeO4����KOH���������л��ܼ���C�л���ᆳ���ˡ�ϴ�ӡ�����õ�����������ؾ��壬ϴ��ʱϴ�Ӽ���ѡ������� ��С���Ϊ��c��
��4��K2FeO4���Խ���ˮ�е�CN������ΪCNO������Ӧ������FeO42������Ԫ�ؼ�̬��+6�۽��͵�+3������Fe(OH)3��CN����̼Ԫ�ؼ�̬��+2�����ߵ�+4������CNO�������ݵ�ʧ�����غ㡢ԭ���غ㡢����غ���ƽ���ӷ���ʽ�����ӷ���ʽΪ��2FeO42��+3CN��+5H2O=2Fe(OH)3��+3CNO��+4OH������С���Ϊ��2 3 5 2 3 4��
��5����������K2FeO4������H2SO4��Һ�л�ת��ΪFe3+��O2�������Һ�в�����K2FeO4����Һ���dz��ɫһ����MnO4������ɫ����С���Ϊ��������dz��ɫ��Һ�еμӹ���ϡH2SO4������Һdz��ɫδ��ȥ��