��Ŀ����
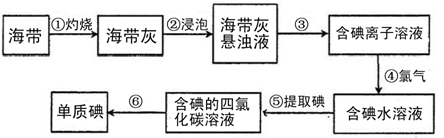
�����к��зḻ�ĵ⣮Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺


����д���пհף�
��1�������������п�֪�������к��еĵ�Ԫ�ص���Ҫ������ʽ��______��
��2����������պ���ʱ������Ҫ���żܡ��������⣬����Ҫ�õ���ʵ��������______
��������������ѡ��������������ñ����ĸ��д�ڿհ״�����
A���ձ� B�������� C������ D���ƾ��� E��������
��3��������ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ������辭������ָ������ʵ��
װ���еĴ���֮����
��______
��______
��______
���������������ʱ��ʹ��ˮԡ��ԭ����______��
��4������ܷ�Ӧ�����ӷ���ʽ��______��������е�ת��������Cl2��˫��ˮ��H2O2������ֱ�д����Ӧ�����ӷ���ʽ��
______��______��
��5�����麣�����Ƿ��е�Ԫ�أ������ڲ���ܺ���У�����ʱ���õ��Լ���______��
��6���������ʣ�������Ϊ�ӵ�ˮ����ȡ����ܼ�����______��
A���������� B��CCl4 C���ƾ� D���ױ�
��7������۵õ�����Һ��ʱ�����ǻ��ǵģ���ͨ��ԭ����______��


����д���пհף�
��1�������������п�֪�������к��еĵ�Ԫ�ص���Ҫ������ʽ��______��
��2����������պ���ʱ������Ҫ���żܡ��������⣬����Ҫ�õ���ʵ��������______
��������������ѡ��������������ñ����ĸ��д�ڿհ״�����
A���ձ� B�������� C������ D���ƾ��� E��������
��3��������ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ������辭������ָ������ʵ��
װ���еĴ���֮����
��______
��______
��______
���������������ʱ��ʹ��ˮԡ��ԭ����______��
��4������ܷ�Ӧ�����ӷ���ʽ��______��������е�ת��������Cl2��˫��ˮ��H2O2������ֱ�д����Ӧ�����ӷ���ʽ��
______��______��
��5�����麣�����Ƿ��е�Ԫ�أ������ڲ���ܺ���У�����ʱ���õ��Լ���______��
��6���������ʣ�������Ϊ�ӵ�ˮ����ȡ����ܼ�����______��
A���������� B��CCl4 C���ƾ� D���ױ�
��7������۵õ�����Һ��ʱ�����ǻ��ǵģ���ͨ��ԭ����______��
��1������۵õ��������ӵ���Һ���ʴ�Ϊ�������ӣ�
��2�����չ�������һ��ʹ�ã��ɣ�������������������Ҫ��������֧��Ȼ��������ż��ϣ����ż�����Ŀռ�žƾ��ƣ��ʴ�Ϊ��CD��
��3�����������ע�������֪��ͼ�г������´����¶ȼ�ˮ�����λ�ô���Ӧ������ƿ֧������ƽ�룻���ձ���û��ʯ�������������ʱ�����Ȳ�����ը�ѣ���������������ˮ�����������ˮӦ���·������Ϸ��������þƾ���ֱ�Ӽ����¶ȱ仯�죬��ˮԡ�����Թ����Լ����ȱȽϾ��ȣ��ʴ�Ϊ�����¶ȼ�ˮ�����λ�ô���Ӧ������ƿ֧������ƽ�룻���ձ���û��ʯ�������������ʱ�����Ȳ�����ը�ѣ���������������ˮ�����������ˮӦ���·������Ϸ������Լ����Ⱦ��ȣ�
��4�������������������¿ɱ�MnO2������Cl2��˫��ˮ��H2O2�������������ӣ��ʴ�Ϊ��2I-+MnO2+4H+=Mn2++I2+2H2O��Cl2+2I-=2Cl-+I2��H2O2+2I-+2H+=I2+2H2O��
��5���������۱���ɫ���ʴ�Ϊ��������Һ��
��6�����������к��в����͵�ϩ�����������ⷢ����Ӧ������ֱ�����;Ϳ����ˣ��ƾ����ֲ㣬�ʴ�Ϊ��AC��
��7�����˵Ĺ��������Ѳ���������������ֽ��ʱ��С�İ���ֽ�����ˣ���ʱ���˳�����Һ�϶��ǻ��ǵģ�����ʱ��ҺҺ��һ��Ҫ������ֽ�ı�Ե����Ȼ��Һ�ͻ������ֽ��ֱ�Ӵ���ֽ��©��֮��Ŀ�϶��������
�ʴ�Ϊ����ֽ�����㵹Һ��ʱ����ҺҺ�������ֽ��Ե��
��2�����չ�������һ��ʹ�ã��ɣ�������������������Ҫ��������֧��Ȼ��������ż��ϣ����ż�����Ŀռ�žƾ��ƣ��ʴ�Ϊ��CD��
��3�����������ע�������֪��ͼ�г������´����¶ȼ�ˮ�����λ�ô���Ӧ������ƿ֧������ƽ�룻���ձ���û��ʯ�������������ʱ�����Ȳ�����ը�ѣ���������������ˮ�����������ˮӦ���·������Ϸ��������þƾ���ֱ�Ӽ����¶ȱ仯�죬��ˮԡ�����Թ����Լ����ȱȽϾ��ȣ��ʴ�Ϊ�����¶ȼ�ˮ�����λ�ô���Ӧ������ƿ֧������ƽ�룻���ձ���û��ʯ�������������ʱ�����Ȳ�����ը�ѣ���������������ˮ�����������ˮӦ���·������Ϸ������Լ����Ⱦ��ȣ�
��4�������������������¿ɱ�MnO2������Cl2��˫��ˮ��H2O2�������������ӣ��ʴ�Ϊ��2I-+MnO2+4H+=Mn2++I2+2H2O��Cl2+2I-=2Cl-+I2��H2O2+2I-+2H+=I2+2H2O��
��5���������۱���ɫ���ʴ�Ϊ��������Һ��
��6�����������к��в����͵�ϩ�����������ⷢ����Ӧ������ֱ�����;Ϳ����ˣ��ƾ����ֲ㣬�ʴ�Ϊ��AC��
��7�����˵Ĺ��������Ѳ���������������ֽ��ʱ��С�İ���ֽ�����ˣ���ʱ���˳�����Һ�϶��ǻ��ǵģ�����ʱ��ҺҺ��һ��Ҫ������ֽ�ı�Ե����Ȼ��Һ�ͻ������ֽ��ֱ�Ӵ���ֽ��©��֮��Ŀ�϶��������
�ʴ�Ϊ����ֽ�����㵹Һ��ʱ����ҺҺ�������ֽ��Ե��

��ϰ��ϵ�д�
�����Ŀ