��Ŀ����
��CuSO4��Һ����μ�KI��Һ���������۲쵽������ɫ����CuI����Һ��Ϊ��ɫ������Ӧ��Ļ�����в���ͨ��S02���壬��Һ�����ɫ�������з�������ȷ���ǣ�������
��������CuSO4��Һ����μ�KI��Һ���������۲쵽������ɫ����CuI��ͭ���Ӿ�����������������Ϊ���ʵ⣬��Һ��Ϊ��ɫ������Ӧ��Ļ�����в���ͨ��S02���壬��Һ�����ɫ���ⵥ�ʺͶ���������ˮ��Һ�з�Ӧ���ɵ⻯������ᣮ
����⣺A���μ�KI��Һʱ����ӦΪ2Cu2++4I-=2CuI��+I2��ת��2mol e-ʱ����2mol��ɫ��������A����
B��ͨ��SO2����Һ�����ɫ��SO2+I2=2H2O=2HI+H2SO4��������SO2�Ļ�ԭ�ԣ���B����
C��ͨ��S02ʱ��S02��I2��Ӧ��SO2+I2=2H2O=2HI+H2SO4��I2������������C����
D�����ݷ�Ӧ2Cu2++4I-=2CuI��+I2��SO2+I2=2H2O=2HI+H2SO4��������ԭ��Ӧ���������������Դ�����������õ�Cu2+��I2��SO2����D��ȷ��
��ѡD��
B��ͨ��SO2����Һ�����ɫ��SO2+I2=2H2O=2HI+H2SO4��������SO2�Ļ�ԭ�ԣ���B����
C��ͨ��S02ʱ��S02��I2��Ӧ��SO2+I2=2H2O=2HI+H2SO4��I2������������C����
D�����ݷ�Ӧ2Cu2++4I-=2CuI��+I2��SO2+I2=2H2O=2HI+H2SO4��������ԭ��Ӧ���������������Դ�����������õ�Cu2+��I2��SO2����D��ȷ��
��ѡD��
���������⿼���˶����������ʵķ����жϣ���������ԭ�Ե�Ӧ�ã�������ԭ��Ӧ�ĸ�������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
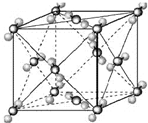 ��2009?��Ǩģ�⣩��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ��������Ӳ��p�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ������ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ�
��2009?��Ǩģ�⣩��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ��������Ӳ��p�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ������ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ�