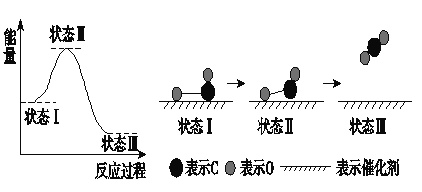
��Ŀ����
����Ŀ��������ѧ֪ʶ����ش��������⣺
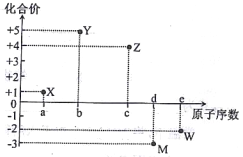
��1��ǰ������Ԫ���У���̬ԭ����δ�ɶԵ���������������������ͬ��Ԫ����______�֡�
��2�������������ڵĵڢ�B��Ԫ�ػ�̬ԭ�ӵ����Ų�ʽ��____________________��
��3��ijԪ�ر���ѧ�ҳ�֮Ϊ������Ԫ���еġ�����֮��������ԭ�ӵ���Χ�����Ų���4s24p4����Ԫ�ص�������_________��
��4���ڼ��Է���NCl3�У�Nԭ�ӵĻ�����Ϊ�D3��Clԭ�ӵĻ��ϼ�Ϊ��1�����Ʋ�NCl3ˮ�����Ҫ������_______________(�ѧʽ)��
��5���ڢ�A���Ԫ�����������ܲ��p�ܼ����пչ�����ʳ�Ϊȱ����Ԫ�ء�����Ľṹʽ�ɱ�ʾΪ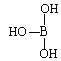 ������������ˮ��1�����������ˮ���ã�ֻ�ܲ���1��H+����д����������ˮ����Һ�����Ե����ӷ���ʽ��_______________________________��
������������ˮ��1�����������ˮ���ã�ֻ�ܲ���1��H+����д����������ˮ����Һ�����Ե����ӷ���ʽ��_______________________________��
��6����Ҫ��д���ɵڶ�����Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӵĻ�ѧʽ������дһ�֣������������_________�������η��� _________ ��
��7���ѱ���Ϊδ����������֪ij�ѵĻ�����TiCl3��6H2O����Է�������Ϊ262.5����λ��Ϊ6��ȡ�þ���26.25�������Һ������������������Һ�����ˣ�ϴ�ӣ���ɣ����أ�����Ϊ28.70�ˣ���þ���Ļ�ѧʽ�ɱ�ʾΪ______________________________��
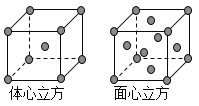
��8���������ľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ���ͼ��ʾ��������������������������������ʵ�ʺ��е�Feԭ�Ӹ���֮��Ϊ_____________�������� ���������ڽ�����ԭ�Ӽ������ͬ���������������������������������ܶ�֮��Ϊ____________��
���𰸡� 5 1s22s22p63s23p63d104s1��zAr�{3d104s1 �� HClO��NH3��H2O H3BO3+ H2O![]() H4BO4- + H+ CH4��CF4 NH3��NF3 ��TiCl��H2O��5��Cl2��H2O 1��2 3
H4BO4- + H+ CH4��CF4 NH3��NF3 ��TiCl��H2O��5��Cl2��H2O 1��2 3![]() /8
/8
�����������⿼����ԭ�ӵĵ����Ų��ͷ��ӹ��ͣ��Լ������ļ���ȣ�ע������������ڽ������֪����������������������������ԭ�ӽ����������ɽ�����⡣
(1) ǰ������Ԫ���У���̬ԭ����δ�ɶԵ���������������������ͬ��Ԫ�� �У���һ���ڵ���Ԫ�أ��ڶ����ڵ�̼Ԫ�غ���Ԫ�أ��������ڵ���Ԫ�أ��������ڵ���Ԫ�ء���5�֡�(2) �������ڵĵڢ�B��Ԫ��Ϊͭ��29��Ԫ�أ����̬ԭ�ӵ����Ų�ʽ 1s22s22p63s23p63d104s1��zAr�{3d104s1 ��(3) ԭ�ӵ���Χ�����Ų���4s24p4����Ԫ��Ϊ34��Ԫ�أ�����Ϊ����(4) �ڼ��Է���NCl3�У�Nԭ�ӵĻ�����Ϊ�D3��Clԭ�ӵĻ��ϼ�Ϊ��1������ˮ���ԭ���������ʷֳ��������ӣ��ֱ��ˮ����������Ӻ����������ӽ�����ɲ����NCl3ˮ��������Ҫ������HClO��NH3��H2O��(5)���������Bԭ�Ӻ���һ�չ������ˮ������������������γ���λ���γ�H4BO4-��ͬʱ����1�������ӣ���������ˮ����Һ�����Ե����ӷ���ʽΪ H3BO3+ H2O![]() H4BO4- + H+ (6) �ɵڶ�����Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӣ�˵������ԭ�ӽײ���Ӷ���4��������������幹�ͣ�������ԭ�Ӳ����¶Ե��Ӷԣ�Ϊ������ķ���̼������������νṹ˵������ԭ�Ӻ���һ���¶Ե��Ӷԣ�ΪNH3��NF3�� (7)��֪ij�ѵĻ�����TiCl3��6H2O����Է�������Ϊ262.5����λ��Ϊ6��ȡ�þ���26.25�˼�0.1mol�����Һ������������������Һ�����ˣ�ϴ�ӣ���ɣ����أ�����Ϊ28.70�ˣ���0.2mol�Ȼ�����˵��������ﻯѧʽ����к������������ӣ�����һ�������������壬����λ����6�����Ի���5��ˮ���������壬1��ˮ�����ǽᾧˮ�������仯ѧʽΪ��TiCl��H2O��5��Cl2��H2O��(8)�������������� ��ԭ�Ӹ�����
H4BO4- + H+ (6) �ɵڶ�����Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӣ�˵������ԭ�ӽײ���Ӷ���4��������������幹�ͣ�������ԭ�Ӳ����¶Ե��Ӷԣ�Ϊ������ķ���̼������������νṹ˵������ԭ�Ӻ���һ���¶Ե��Ӷԣ�ΪNH3��NF3�� (7)��֪ij�ѵĻ�����TiCl3��6H2O����Է�������Ϊ262.5����λ��Ϊ6��ȡ�þ���26.25�˼�0.1mol�����Һ������������������Һ�����ˣ�ϴ�ӣ���ɣ����أ�����Ϊ28.70�ˣ���0.2mol�Ȼ�����˵��������ﻯѧʽ����к������������ӣ�����һ�������������壬����λ����6�����Ի���5��ˮ���������壬1��ˮ�����ǽᾧˮ�������仯ѧʽΪ��TiCl��H2O��5��Cl2��H2O��(8)�������������� ��ԭ�Ӹ�����![]() =2����������������ʵ�ʺ��е���ԭ�Ӹ�����
=2����������������ʵ�ʺ��е���ԭ�Ӹ�����![]() =4���������������������������������ʵ�ʺ��е���ԭ�Ӹ���֮��Ϊ1��2�������������о������ⳤΪx����ԭ�ӵ�ֱ��ΪA����3x2=(2A)2 �����x=
=4���������������������������������ʵ�ʺ��е���ԭ�Ӹ���֮��Ϊ1��2�������������о������ⳤΪx����ԭ�ӵ�ֱ��ΪA����3x2=(2A)2 �����x= ![]() ����ԭ��ֱ��ΪA�������侧�����ΪR3�����������о����ĶԽ���Ϊ2A����߳�Ϊ
����ԭ��ֱ��ΪA�������侧�����ΪR3�����������о����ĶԽ���Ϊ2A����߳�Ϊ![]() ���侧�����Ϊ
���侧�����Ϊ![]() �������������ܶ��������������ܶ�֮��Ϊ
�������������ܶ��������������ܶ�֮��Ϊ![]() =3
=3![]() /8��
/8��

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д�����Ŀ�����û�ѧԭ���Է�������ˮ������������̼��������ʵ����ɫ�������������ã��Թ�����̬��������Ҫ���塣
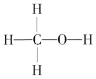
(1)ȼú�����е�CO2��ת��Ϊ��������ԭ�ϡ������Դ�״�(CH3OH���״��Ľṹʽ��ͼ)��
3H2(g)+CO2(g) ![]() CH3OH (g) + H2O(g) ��H
CH3OH (g) + H2O(g) ��H
����֪��
��ѧ�� | C-H | C-O | C=O | H-H | O-H |
����/KJ/mol | 412 | 351 | 745 | 436 | 462 |
���H = _________________
(2)��2 L�ܱ������У�800 ��ʱ��Ӧ2NO(g)��O2(g) ![]() 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
�ٵ���ƽ��ʱNO��ת����Ϊ_________��
����O2��ʾ��0��2 s�ڸ÷�Ӧ��ƽ������v��____________________��
������ͼ��ʾ����ʾNO2�仯���ߵ���________��
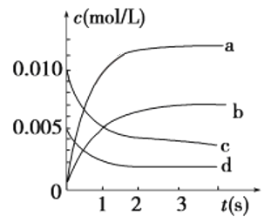
����˵���÷�Ӧ�Ѵﵽƽ��״̬����________(�����)��
A��v(NO2)��2v(O2) B��������ѹǿ���ֲ���
C��v��(NO)��2v��(O2) D�������ڵ��ܶȱ��ֲ���
(3)ij��Ӧ�����ܱ������н��У� ��0~3�����ڸ����ʵ����ı仯�������ͼ��ʾ
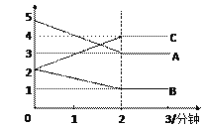
�÷�Ӧ�ĵĻ�ѧ����ʽΪ________________��